Fig. 3.
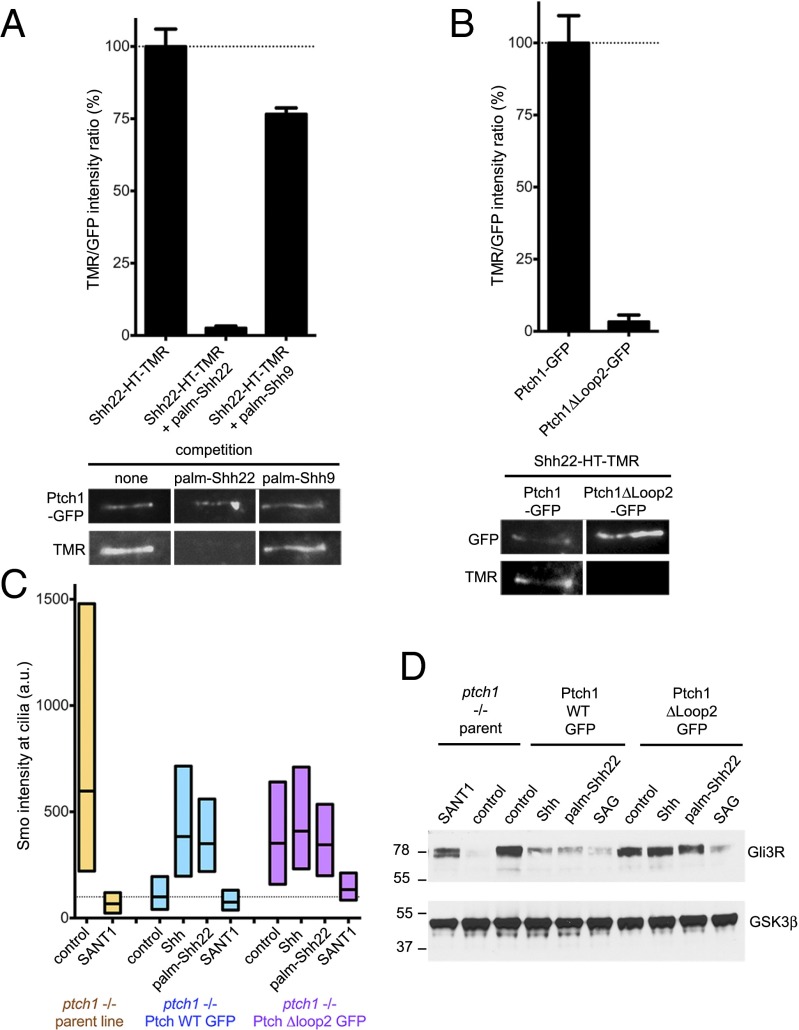
Ptch1 binding is required for signaling by palmitoylated Shh peptide. (A) Binding of Shh22-HT-TMR to cilia in Ptch1-null cells rescued with Ptch1-eGFP was measured by live imaging in the absence or presence of palm-Shh22 or palm-Shh9 (5 μM). Graph shows the ratio of ciliary TMR and GFP fluorescence. Error bars represent SE (n > 5 cilia). Representative images are shown below the graph. Palm-Shh9 does not compete binding of Shh22-HT to Ptch1-eGFP, in contrast to palm-Shh22. (B) As in A, but with Ptch1-null cells rescued with Ptch1-eGFP or Ptch1Δloop2-eGFP. Shh22-HT-TMR does not bind Ptch1Δloop2-eGFP. (C) Ptch1-null cells, rescued or not with Ptch1-eGFP or Ptch1Δloop2-eGFP, were incubated with control media, Shh, palm-Shh22 (5 μM), or SANT1 (1 μM), and endogenous Smo localization to cilia was measured by immunofluorescence and automated image analysis (n > 300 cilia). Smo is constitutively at cilia in Ptch1-null cells, which is reversed by Ptch1-eGFP, and partially by Ptch1Δloop2-eGFP. Palm-Shh22 and Shh do not cause Smo accumulation in cilia in cells rescued with Ptch1Δloop2-eGFP, in contrast to Ptch1-eGFP. In all conditions, Smo recruitment to cilia is blocked by SANT1. (D) As in C, but cells were incubated with control media, Shh, palm-Shh22 (5 μM), SAG (1 μM), or SANT1 (1 μM), and endogenous Gli3R was measured by immunoblotting. GSK3β served as loading control. Ptch1-null cells have low Gli3R levels, indicative of constitutive Hh signaling, which is reversed by Ptch1-eGFP, Ptch1Δloop2-eGFP, or SANT1. Cells expressing Ptch1Δloop2-eGFP do not respond to Shh and palm-Shh22, but respond to SAG; in contrast, cells expressing Ptch1-eGFP respond to all three.