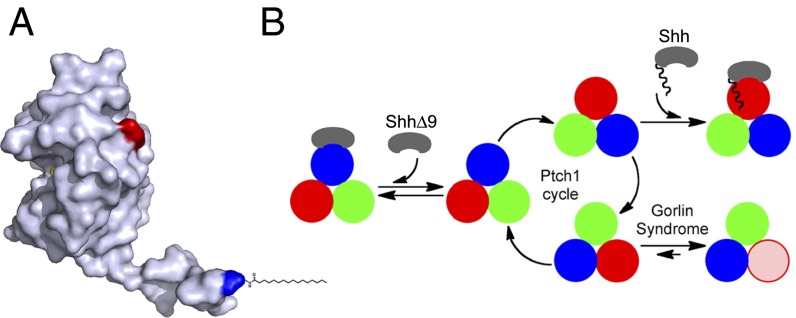
Fig. 7.
Model for Ptch1 inhibition by Shh and by oncogenic mutations. (A) Crystal structure of human Shh (21), showing the N-terminal peptide protruding from the globular part of the protein. The N and C termini are colored blue and red, respectively. The palmitoyl residue was added manually. Shh can be divided into two parts: a short, palmitoylated N-terminal peptide that inhibits Ptch1 via a low-affinity binding site and a globular part that causes Ptch1 internalization via a high-affinity binding site. (B) Speculative model for Ptch1 function and inhibition. Although its oligomeric structure is unknown, Ptch1 is shown as a homotrimer undergoing conformational cycling (different conformations are shown in red, blue, and green) by analogy to bacterial RND pumps. This cycle is proposed to be required for Hh pathway-suppressing activity of Ptch1, perhaps via a small-molecule Smo modulator. Shh binds Ptch1 and, via its palmitoylated N-terminal portion, interrupts cycling by conformational trapping. ShhΔ9 binds Ptch1 with high affinity but cannot interrupt cycling and is thus inactive. Oncogenic Ptch1 mutants that cause GS adopt a conformation (pink) defective in interaction with the palmitoylated N-terminal portion of Shh. This conformational trapping could explain reduced activity of these mutants.