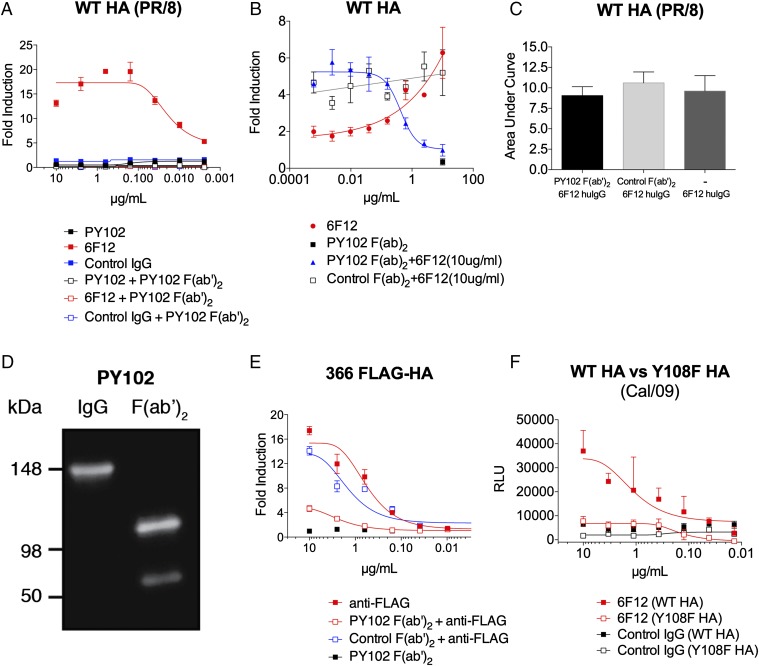
Fig. 3.
Inhibition of HA binding to its sialic acid receptor prevents FcγR-mediated effector function induced by HA stalk mAbs. HEK 293T cells were transfected with WT HA (PR/8) (A–C), 366 FLAG-HA (E), WT HA (Cal/09) (F), or a mutant HA with a point mutation in the receptor binding site. Y108F HA (Cal/09) served as FcγR-mediated effector targets for the indicated mAbs. A genetically modified Jurkat cell line expressing the murine FcyRIV with an inducible luciferase reporter gene was used to determine the induction of antibody-mediated effector activity. (A) Induction of FcγR-mediated effector function by a stalk-specific mAb, 6F12, was inhibited by blocking the sialic acid-binding site of the globular head of HA with coincubation of a constant amount (10 µg/mL) of head-specific PY102 F(ab′)2. (B) FcγR-mediated effector function by a stalk-specific mAb, 6F12 (10 µg/mL), was inhibited in a dose- dependent manner with the addition of a serial dilution of PY102 F(ab′)2 (starting concentration of 10 µg/mL, diluted fourfold) (C) Coincubation of a constant amount (10 µg/mL) of humanized [murine F(ab′)2, human Fc] 6F12 with variable amounts of PY102 or control F(ab′)2 (starting concentration of 10 µg/mL, fourfold dilution) did not prevent 6F12 from binding to the stalk region in a cell-based ELISA. (D) Purified preparations (100 ng) of full-length and F(ab′)2 of PY102 were resolved in an SDS/PAGE gel (in nonreducing conditions) and assessed by Western blot analysis. An anti-mouse F(ab′)2-specific secondary antibody conjugated to HRP was used to visualized the antibody isoforms. (E) Induction of an anti-FLAG mAb against 366 FLAG-HA was also inhibited with a constant amount of PY102 F(ab′)2 (10 µg/mL). (F) A stalk-specific mAb, 6F12, has reduced ability to induce effector activity against Y108F HA (Cal/09) compared with WT HA (Cal/09). Full-length mAbs in A and E were tested at a starting concentration of 10 µg/mL and were serially diluted fourfold, whereas the F(ab′)2 in A and E were coincubated at a constant concentration of 10 µg/mL. The F(ab′)2 in B was added at a starting dilution of 10 µg/mL and was diluted fourfold. An H3-specific mAb, XY102, was used as control IgG in A and F, and the control F(ab′)2 in B and E was generated from a pan-H3 mAb, 9H10. The area under the curve in C was calculated using GraphPad Prism 5 from ELISA values read at 492 nm. A nonlinear regression best-fit curve was generated for each dataset using GraphPad Prism 5. RLU, relative luminescence units. Error bars represent SEM. Results are from one of two independent experiments.