Abstract
Clinical progression over time and cytokine profiles have not been well defined in patients with Middle East respiratory syndrome coronavirus (MERS-CoV) infection. We included 17 patients with laboratory-confirmed MERS-CoV during the 2015 outbreak in Korea. Clinical and laboratory parameters were collected prospectively. Serum cytokine and chemokine levels in serial serum samples were measured using enzyme-linked immunosorbent assay. All patients presented with fever. The median time to defervescence was 18 days. Nine patients required oxygen supplementation and classified into severe group. In the severe group, chest infiltrates suddenly began to worsen around day 7 of illness, and dyspnea developed at the end of the first week and became apparent in the second week. Median time from symptom onset to oxygen supplementation was 8 days. The severe group had higher neutrophil counts during week 1 than the mild group (4,500 vs. 2,200/µL, P = 0.026). In the second week of illness, the severe group had higher serum levels of IL-6 (54 vs. 4 pg/mL, P = 0.006) and CXCL-10 (2,642 vs. 382 pg/mL, P < 0.001). IFN-α response was not observed in mild cases. Our data shows that clinical condition may suddenly deteriorate around 7 days of illness and the serum levels of IL-6 and CXCL-10 was significantly elevated in MERS-CoV patients who developed severe diseases.
Keywords: MERS, Coronavirus, Clinical Progression, Cytokine, Chemokine, IFN-α, IL-6, CXCL-10, Severity
Graphical Abstract
INTRODUCTION
Middle East Respiratory Syndrome coronavirus (MERS-CoV) infection is a highly lethal respiratory disease that has recently emerged in the Middle East region. It was first reported in a patient who died of progressive pneumonia and renal failure in a hospital in Jeddah, Saudi Arabia in June 2012 (1). As of April 14, 2016, there have been 1,714 laboratory confirmed cases of human infection with MERS-CoV, including at least 618 deaths (2).
In a recent outbreak in Korea, an infected business man returning from the Middle East had introduced MERS-CoV into the country (3). The outbreak was amplified by superspreading events in hospitals and resulted in 186 laboratory-confirmed cases with 38 fatalities, the largest outbreak outside the Arabian Peninsula (4,5). Factors contributing to the large hospital outbreaks included delay in diagnosis of the index case, overcrowding in emergency departments, movements of patients prior to diagnoses, and suboptimal infection prevention and control (6). During the outbreak, almost 17,000 individuals with an epidemiologic risk were quarantined for monitoring, and all the laboratory-confirmed cases were admitted to the hospitals for isolation as soon as the diagnoses were made. Consequently, the Korean outbreak provided a unique opportunity to conduct prospective studies on MERS-CoV infection.
In human infection with highly virulent respiratory viruses, such as avian influenza H5N1, H7N9, and Severe Acute Respiratory Syndrome (SARS) coronavirus, immunopathogenesis caused by hyper-induction of proinflammatory cytokines (also known as hypercytokinemia or cytokine storm) may play an important role in the disease progression and the ultimate mortality (7,8,9). To better understand immunopathogenesis of MERS-CoV infection, we assessed host immune responses to and viral kinetics of MERS-CoV infection. We recently reported the viral shedding kinetics (10) and antibody response kinetics (11). However, the innate immune response to MERS-CoV infection and the role of most cytokines in disease severity remains unclear. Here, we report detailed clinical course and cytokine profiles in patients with MERS-CoV infection. We also evaluated the association between the cytokine profiles and severity of illness.
MATERIALS AND METHODS
Patient population
During the outbreak of MERS-CoV in Korea, all laboratory-confirmed cases were admitted to the government-designated hospitals regardless of their severity of illness. They were discharged from the hospitals when two consecutive daily real-time reverse transcription (rRT)-PCR were all negative after 48 hours of the resolution of all clinical symptoms. The laboratory diagnosis was made by WHO guideline (12). We included all patients admitted to three Seoul National University (SNU) affiliated hospitals (SNU Hospital, SNU Bundang Hospital, and SNU Boramae Medical Center). We excluded those patients who were admitted 14 days after symptom onset. These patients were the same as those of our studies reported previously (10,11).
Data collection
Board certified infectious disease specialists in each hospital prospectively documented clinical symptoms, physical examinations, and laboratory values using standardized case recording form. For the patients who were transferred from other hospitals, the medical records and chest radiographs of the patients at the hospital were also reviewed. Serum samples were collected at serial interval and stored at −70°C.
The patients were categorized into severe or mild group depending upon oxygen supplementation requirements. The severe group required oxygen supplementation to maintain arterial saturation above 90%.
Cytokine assay
Cytokines levels in serum were assessed by enzyme-linked immunosorbent assay (ELISA) using VeriKine ELISA kits (PBL Assay Science, Piscataway, NJ, USA) for interferon α (IFN-α) and interferon β (IFN-β), and Human Duoset kits (R&D Systems, Minneapolis, MN, USA) for interferon γ (IFN-γ), interleukin 1α (IL-1α), interleukin 6 (IL-6), interleukin 10 (IL-10), interleukin 12p70 (IL-12p70), interleukin 12/interleukin 23p40 (IL-12/IL-23p40), interleukin 17 (IL-17), C-X-C motif chemokine 10 (CXCL-10), tumor necrosis factor α (TNF-α), transforming growth factor β3 (TGF-β3). The assay ranges of each ELISA kit are shown in Supplementary Table 1.
Statistical analysis
Mann-Whitney U test was used to analyze the difference between two groups and the Spearman’s rank correlation was used to determine the correlation between variables. Statistical analyses were performed with IBM SPSS Statistics software 22.0 (SPSS Inc., Chicago, IL, USA). All significance tests were two-sided.
Ethics statement
The institutional review board at Seoul National University Hospital reviewed the study protocol and provided study approval. The board waived the requirement for written consent (IRB registration number 1506-093-681).
RESULTS
Clinical features
A total of 17 patients were included in the study. The demographic and clinical characteristics of the patients are shown in Table 1. The median incubation period was 7 days (range, 2 to 14 days) and the median interval from symptom onset to isolation unit was 5 days (range, 2 to 11 days).
Table 1. Demographic and clinical profiles of the study participants.
| Patient ID | Sex/Age, yr | Underlying disease (Pre-existing infection) | Incubation period | CXR infiltrates | Oxygen therapy | Mechanical ventilator | Steroid (Duration) | Antiviral therapy (Duration) | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| A | M/38 | 2 days | Yes (D6−) | Yes | Yes | Yes (D14–D39) | IFN (D5, D10, D17) + RBV (D5, D10–D20) | Transfer (D61) | |
| B | M/65 | 9 days | Yes* (D11−) | Yes | Yes | Yes (D14–D20) | IFN (D11, D18) + RBV (D11) + LPV/r (D11–D20) | Died (D142) | |
| C | M/55 | 11 days | Yes* (D5−) | Yes | Yes | No | IFN (D6, D13) + RBV (D6–D14) + LPV/r (D6–D16) | Discharge (D26) | |
| D | M/35 | (Bacterial pneumonia) | 6 days | Yes (D5−) | Yes | Yes | Yes (D11–D23) | IFN (D10, D17) + RBV (D10–D23) | Discharge (D33) |
| E | F/79 | CHD, Dementia, CKD, Bladder cancer | 12 days | Yes* (D2−) | Yes | Yes† | Yes (D11-D17) | IFN (D12) + RBV (D12–D13) + LPV/r (D12–D13) | Died (D17) |
| F | M/55 | DM, CPD, (Lung abscess) | 5 days | Yes* (D1−) | Yes | No | Yes (D14–D16) | No | Discharge (D31) |
| G | M/56 | 6 days | Yes* (D4−) | Yes | No | No | IFN (D8, D15) + RBV (D8–D14) + LPV/r (D8-D17) | Discharge (D40) | |
| H | M/71 | DM, CVA (Aspiration pneumonia) | 6 days | Yes* (D2−) | Yes | No | No | No | Discharge (D38) |
| I | F/77 | DM, Asthma | 7 days | Yes* (D11-) | Yes | No | No | No | Discharge (D18) |
| J | M/76 | DM, CHD | 10 days | Yes* (D6−) | No | No | No | No | Discharge (D28) |
| K | M/59 | CHD | 2 days | Yes* (D9−) | No | No | No | IFN (D9) + RBV (D9–D13) | Discharge (D19) |
| L | F/56 | 4 days | Yes* (D12−) | No | No | No | No | Discharge (D21) | |
| M | M/56 | DM, CHD, CLD (Tuberculosis) | 7 days | Yes* (D3−) | No | No | No | No | Discharge (D16) |
| N | F/54 | 7 days | Yes* (D3−) | No | No | No | No | Discharge (D21) | |
| O | M/46 | 9 days | Yes (D9−) | No | No | No | No | Discharge (D12) | |
| P | M/35 | 11 days | No | No | No | No | IFN (D4) + RBV (D4) | Discharge (D14) | |
| Q | M/52 | DM (Liver abscess) | 14 days | Yes* (D2−) | No | No | No | IFN (D2, D8) + RBV (D2) + LPV/r (D2–D11) | Discharge (D21) |
CXR = chest X-ray, CHF = chronic heart disease, CKD = chronic kidney disease, DM = diabetes mellitus, CPD = chronic pulmonary disease, CVA = cerebrovascular accident, CLD = chronic liver disease, IFN = pegylated interferon alpha-2a, RBV = ribavirin, LPV/r = ritonavir boosted lopinavir.
*The first chest radiography showed infiltrates; †This patient refused intubation.
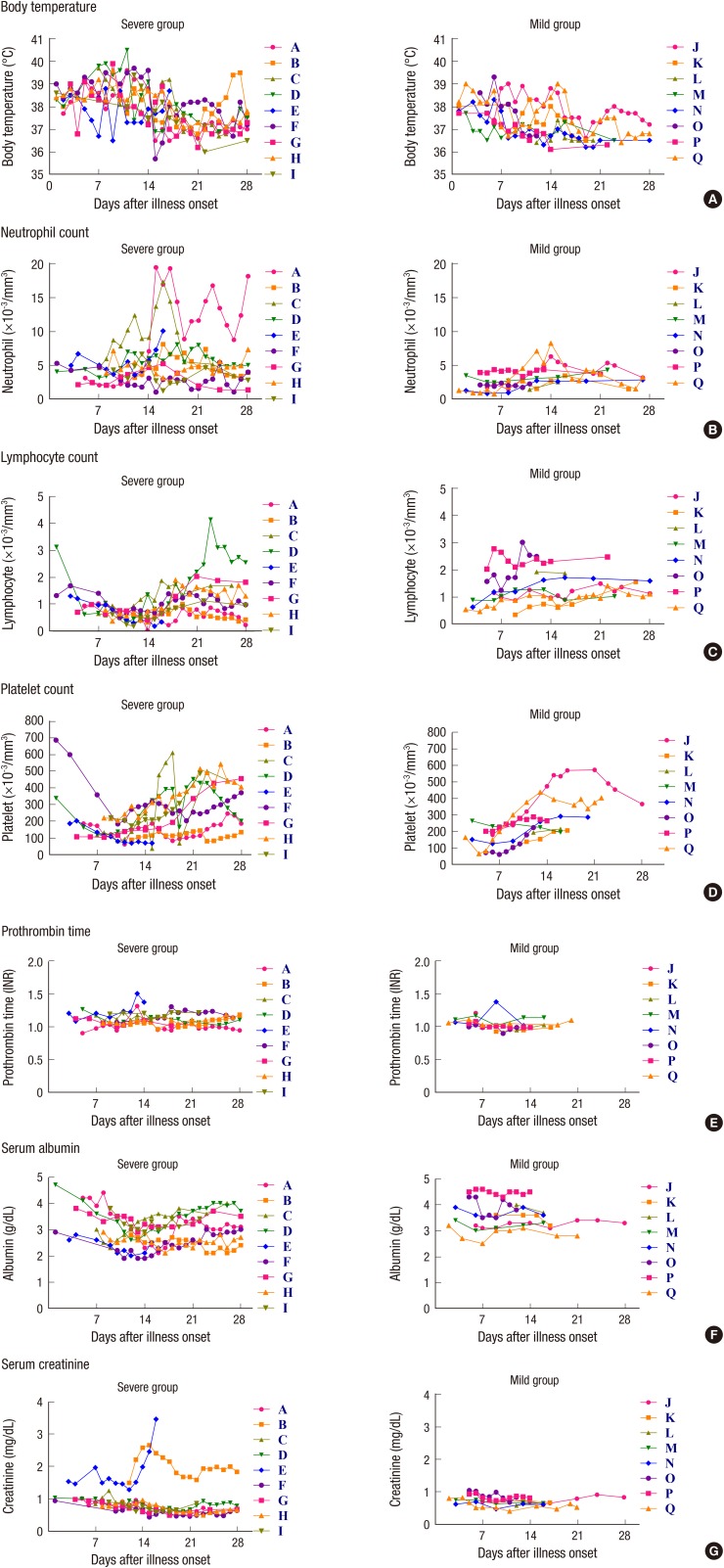
The clinical symptoms during the first week of illness were fever (100%), cough (75%), malaise (75%), anorexia (70%), chilling (45%), diarrhea (33%), dyspnea (29%), nausea (20%), and sore throat (15%). Changes in frequency of these symptoms over time after illness onset are partly shown in Supplementary Fig. 1. The median time to defervescence was 18 days (Fig. 1A). Dyspnea developed at the end of the first week, and became apparent in the second week.
Fig. 1.
Changes of body temperature and laboratory parameters over time after symptom onset in the patients with MERS-CoV infection. Shown are body temperature (A), neutrophil count (B), lymphocyte count (C), platelet count (D), serum albumin (E), prothrombin time (F), and serum creatinine (G). The severe cases were the patients who required oxygen supplementation or mechanical ventilation, whereas the mild cases were those who did not required oxygen supplementation.
Nine patients (Patient A to I) needed oxygen supplement (Table 1). The median time from symptom onset to oxygen supplementation was 8 day of illness (range, 6 to 12 day of illness). The median time to hospital discharge was 21 day of illness (range, 12 to 142 day of illness).
Laboratory changes are shown in Fig. 1B to 1G and Supplementary Table 2. The severe group had higher neutrophil counts during week 1 than the mild group (4,500 vs. 2,200/µL, P = 0.026) (Fig. 1B). There was overall lymphopenia and it was marked (lymphocyte counts less than 1,000/µL) from the mid-first week to the mid-third week (Fig. 1C). Platelet counts below 100,000/µL rarely developed in both severe and mild groups, and there were no clinically significant abnormalities in coagulation panel (prothrombin time and activated partial thromboplastin time) (Fig. 1D and 1E). Hypoalbuminemia developed in second week especially in severe group (Fig. 1F). The median lowest level of serum albumin was significantly low in severe group (2.1 g/dL in severe group and 3.4 g/dL in mild group, P = 0.001). Two patients (Patient B and E) had elevated serum creatinine level. Patient B had no underlying disease such as diabetes, hypertension or chronic renal disease. He did not receive any nephrotoxic drugs. Patient E had metastatic bladder cancer and her renal failure was due to metastatic urinary obstruction.
Cytokine profiles
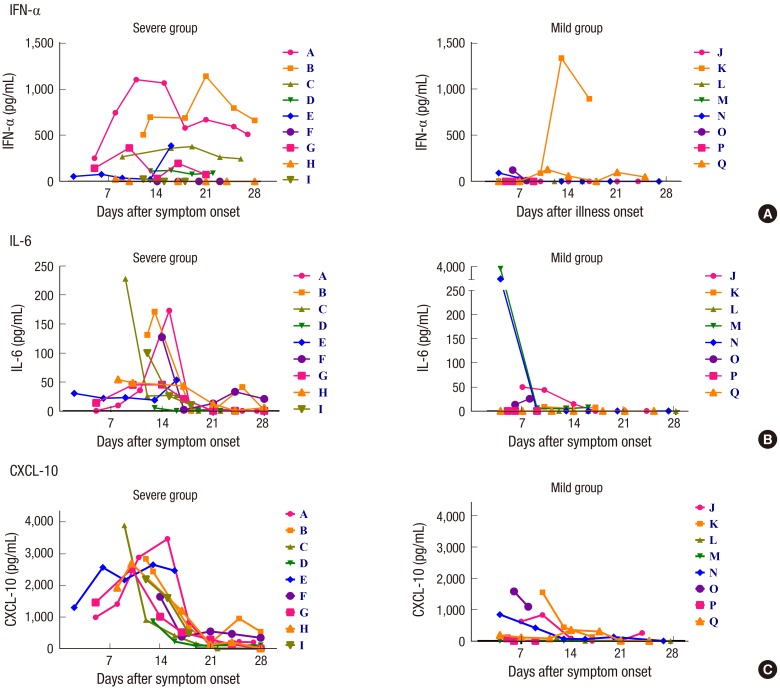
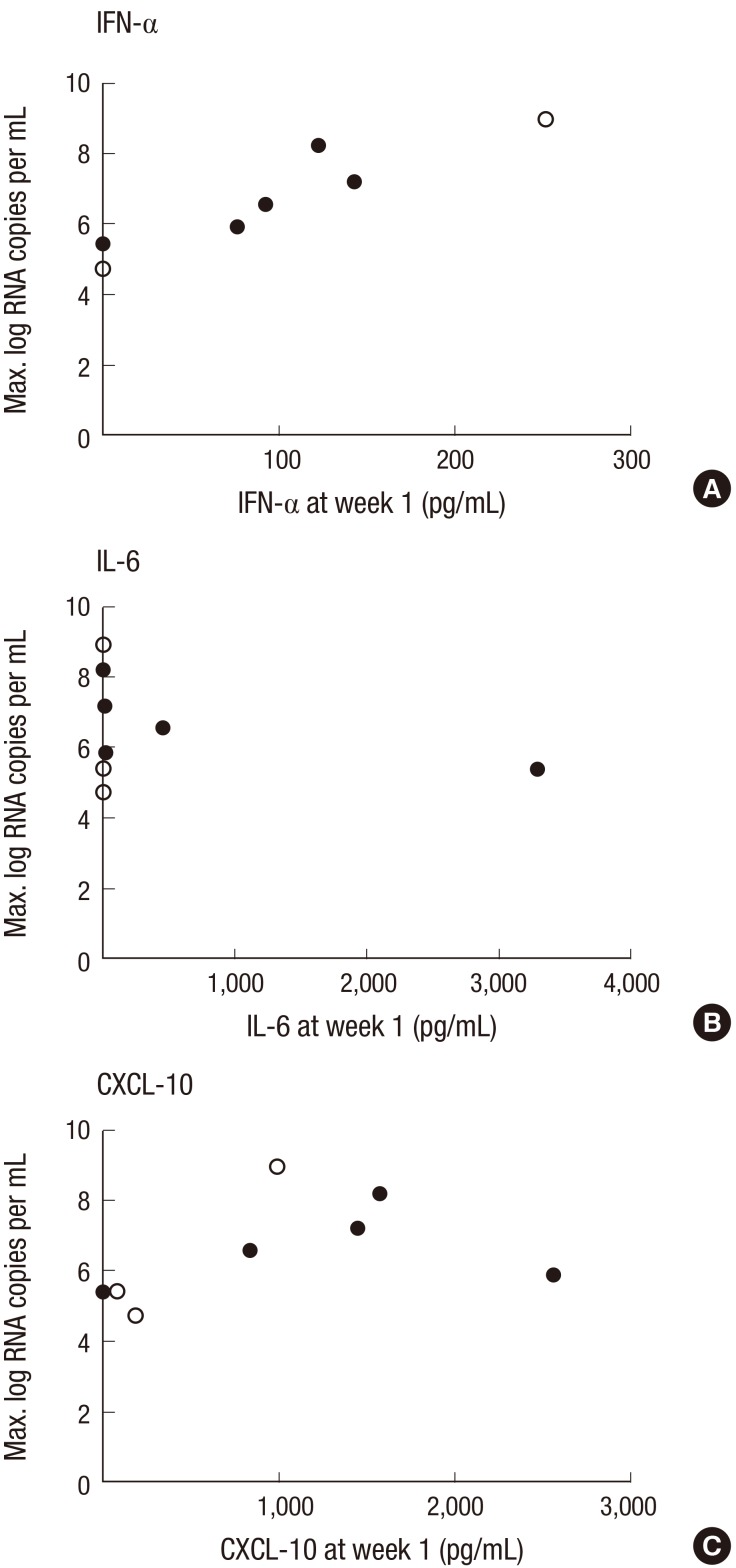
IFN-γ, IL-10, IL-12p70, IL-12/IL-23p40, and IL-17 were not detected in our experiment setting in serum of any patient during the course of disease. IFN-β, TNF-α, TGF-β3, and IL-1α were detected in a few patients, but they did not show any significant relationship with the clinical course or severity of illness. The serum levels of IFN-α increased during the course of disease in some of severe cases, but pegylated IFN-α2a was injected in six among nine severe cases. There was no apparent IFN-α response in the mild group except one patient who received IFN-α injection (Fig. 2A). When comparing the peak viral load of MERS-CoV in sputum and the serum levels of cytokines or chemokines at the first week of illness, a significant correlation was observed for IFN-α (ρ = 0.983, P = 0.001) (Fig. 3A).
Fig. 2.
Changes of the serum levels of cytokine and chemokine over time after symptom onset in the patient with MERS-CoV infection. IFN-α (A), IL-6 (B), and CXCL-10 (C) increased during the course of disease, especially in the severe group. The following patients received pegylated IFN-α2a injection: Patient A (Day 5, 10, 17), Patient B (Day 11, 18), Patient C (Day 6, 13), Patient D (Day 10, 17), Patient E (Day 12), Patient G (Day 8, 15), Patient K (Day 9), Patient P (Day 4), Patient Q (Day 2, 8).
Fig. 3.
Correlation between serum cytokines and chemokine concentration at the first week of illness and the peak MERS-CoV viral load in respiratory specimens. Hollow circles mean the values measured after the administration of pegylated IFN-α2a. Spearman’s rank correlation coefficient was used to assess correlations. (A) IFN-α, ρ = 0.938, P = 0.001; (B) IL-6, ρ = −0.320, P = 0.440; (C) CXCL-10, ρ = 0.419, P = 0.301.
The serum levels of IL-6 and CXCL-10 increased and usually peaked between 7 and 14 days (Fig. 2B and 2C). The peak of CXCL-10 was higher and more delayed by about one week in the severe group than in the mild group (Fig. 2C). In the second week of illness, the serum level of CXCL-10 was significantly higher in the severe group (median 2,642 pg/mL; range 859–3,884) than in the mild group (median 382 pg/mL; range 0–1,545, P < 0.001). The serum level of IL-6 was also significantly higher in the severe group (median 54 pg/mL; range 5–228) than in the mild group (median 3 pg/mL; range 0–44, P = 0.006) (Supplementary Fig. 3).
DISCUSSION
Our study presents the clinical progression and cytokine profiles of MERS-CoV infection. During the Korean outbreak of MERS-CoV, almost all close contacts were under active monitoring and as soon as the PCR tests were MERS-CoV positive, they were admitted to the hospitals designated by the government. Therefore, we could identify the exact dates of exposure and symptom onset, and were able to conduct this prospective study.
The clinical manifestations of MERS-CoV infection range from asymptomatic infection to fatal pneumonia (13). In this study, common presenting symptoms were fever, chills, and cough. Upper respiratory symptoms such as rhinorrhea and sore throat were rare. Previous studies reported that pneumonia progresses very rapidly, with a median time to the need for mechanical ventilation of 7 days (range, 3 to 11 days) (14,15). In this study, however, most patients had pneumonia in week 1, but their symptoms were not so severe that they did not feel they needed to stay at home (“walking pneumonia”). Dyspnea requiring oxygen supplementation and sudden progression of pneumonia usually developed during the second week. Similar to SARS (16), the median time from symptom onset to ventilator support was 11 days. Because of this clinical progression of initial mild symptoms followed by sudden clinical deterioration, patients with MERS-CoV infection may present at emergency room (ER). Therefore, ER department should prepare for the early detection and isolation of a possible MERS case with appropriate infection prevention and control methods.
Common laboratory abnormalities were anemia, lymphopenia, thrombocytopenia, and elevated alanine aminotransferase level. The laboratory changes were mild during the first week, which were not severe even in the patients with progressive pneumonia. Prolongation of activated partial-prothrombin time rarely developed, even when pneumonia progressed to respiratory failure. Low albumin level was a predictor of severe pneumonia in MERS-CoV infection (17). We also noted that development and progression of pneumonia was associated with decrease in serum albumin level.
Acute renal impairment was initially reported as a characteristic feature of MERS (1). Subsequent studies from Saudi Arabia reported almost half of the patients with MERS required renal replacement therapy (17,18,19). In this study, excluding Patient E who had metastatic bladder cancer with urinary obstruction, only one patient showed acute kidney injury. This striking difference in the incidence of renal impairment is likely due to the low prevalence of underlying conditions such as chronic renal diseases, diabetes and hypertension in our population.
Evasion from immune system in humans is critical for MERS-CoV to survive and replicate in the host. MERS-CoV is known to inhibit recognition, delay IFN induction, and sequester IFN stimulated gene production until after peak viral titers had been achieved (20). In a fatal MERS case, the patient could not induce the expression of key receptors, retinoic acid-inducible gene 1 (RIG-1) and melanoma differentiation-associated protein 5 (MDA-5) in response to MERS-CoV infection (21). This resulted in a decreased expression of IFN regulatory factor 3 (IRF-3) and IRF-7 leading to a decreased expression of IFN-α. In our study, the serum levels of IFN-α in the severe MERS group was more frequently high than the mild group, even if we could not demonstrate any statistical significance. To the contrary, there was no apparent IFN-α response in the mild group (Fig. 2A). The level of IFN-α was also correlated with the log value of maximal RNA copies (Fig. 3A). Taken together, IFN-α seems to surge during the clinical course of MERS in patients having severe disease with high viral loads but not in patients having mild disease. The absence of INF-α surge in severe cases of disease was related to worse outcome in previous study (21), but we could not conclude the association of outcome with the serum level of IFN-α due to a limited number of fatal cases. Moreover, it is possible that the levels of serum INF-α merely reflected the level of exogenously injected pegylated IFN-α2a in this study. Therefore, the results of levels of serum INF-α should be read cautiously.
While IL-6 is one of the important proinflammatory cytokines having diverse biological activities in many clinical situations including respiratory viral infections, the change of serum IL-6 level during MERS-CoV infection has not been clearly documented yet. Increases in serum IL-6 during early phase of respiratory viral infections was revealed in human cases of severe seasonal influenza (22), pandemic 2009 A/H1N1 influenza pneumonia (23), and fatal A/H7N9 avian influenza (8,24). In case of SARS-CoV infections, elevated levels of IL-6 subsequent to the peak viral replication were observed in all of 8 pneumonia cases included (13). IL-6 was induced in MERS-CoV-infected human monocyte-derived macrophages (25) and was significantly upregulated in the lung lesions of the MERS-CoV-infected animals (26). In our study, overall IL-6 levels were not significantly higher in the severe MERS group than in the mild group. However, IL-6 levels in the severe MERS group were higher during the 2nd and the 3rd week after symptom onset with statistical significance when we analyzed a temporal trend (Supplementary Fig. 2).
CXCL-10, also known as IFN-γ-inducible protein 10 (IP-10), is a chemoattractant which is expressed in inflammed bronchial epithelial cells in response to IFN-γ. CXCL-10 seems to play an important role in severe respiratory viral illnesses. Uncontrolled durable secretion of CXCL-10, after the first week following the onset of symptoms was associated with poor outcome in a severe MERS case (21). High serum level of CXCL-10 was also documented in other severe respiratory viral illnesses such as severe A/H7N9 avian influenza (24), A/H5N1 avian influenza (7), severe seasonal influenza (22), and SARS (9). In this study the levels of serum CXCL-10 were significantly higher in the severe MERS group than in the mild group in the second and third week of illness (Supplementary Fig. 2). During the clinical course, the serum levels of CXCL-10 and IL-6 showed a peak around second week after symptom onset in the severe group (Fig. 2C). This dynamic in proinflammatory cytokine response shows a good temporal correlation with chest infiltrates showing a peak at second weeks after symptom onset in severe MERS cases. Therefore, our study suggests that elevated levels of serum IL-6 and CXCL-10 may be associated with the severity of pneumonia in MERS.
This study has some limitations. Small numbers of patients were included and five patients had concurrent infectious diseases, which might affect the clinical course and laboratory features of MERS-CoV infection. Nine patients received pegylated INF-α2a, which could affect serum level of INF-α and other cytokine profiles through cytokine feedback cascades. However, in each group the IL-6 and CXCL-10 profiles were similar regardless of the administration of pegylated INF-α2a. Also subset analysis including the patients who did not received interferon therapy showed similar trends (Supplementary Fig. 4). In spite of these limitations, this study has strengths in that we collected clinical data and serum samples serially in prospective manner and diverse cytokine or chemokine levels were measured in this newly emerging disease.
In conclusion, patients with MERS-CoV infection present with “walking pneumonia” during the first week of illness, which may deteriorate suddenly around day 7 of illness. The serum levels of IL-6 and CXCL-10 were significantly elevated in severe cases during the second week of illness.
ACKNOWLEDGMENT
We are grateful to all the patients for participating in the study and the staffs at the Wide River Institute of Immunology, Seoul National University College of Medicine, who provided technical support in detecting the serum cytokine and chemokine levels.
Footnotes
Funding: This work was supported by a grant from the Korean Healthcare Technology R & D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI15C3227).
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conception and design: Oh MD, Kim ES, Choe PG, Park WB. Acquisition of data: Kim ES, Choe PG, Park WB, Oh HS, Kim EJ, Nam EY, Na SH, Kim MS, Song KH, Bang JH, Park SW. Analysis and interpretation of data: Oh MD, Kim ES, Choe PG, Park WB, Song KH, Bang JH, Park SW, Kim HB, Kim NJ. Manuscript preparation: Oh MD, Kim ES, Choe PG. Manuscript approval: all authors.
Supplementary Materials
The assay range of ELISA kit
Serial clinical and laboratory results during four weeks from the onset of illness.
Clinical symptoms over the course of MERS-CoV infection.
Shown are the changes in clinical symptoms (fever, sore throat, rhinorrhea, cough, sputum, and diarrhea) during the course of diseases. Clinical symptoms before admission were assessed retrospectively via interview on admission and clinical symptoms after admission to study hospitals were assessed prospectively until discharge. Upper respiratory symptoms such as rhinorrhea and sore throat were rare during the course of disease.
Comparison of cytokine and chemokine levels between the severe group and the mild group.
Hollow circles mean the values measured after the administration of pegylated IFN-α2a.
Changes of the serum levels of cytokine and chemokine over time after symptom onset in the patient with MERS-CoV infection, excluding the measurement values after administration of pegylated interferon alpha-2a.
Comparison of cytokine and chemokine levels between the severe group and the mild group, excluding the measurement values after administration of pegylated interferon alpha-2a.
References
- 1.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV) - Saudi Arabia: disease outbreak news, 14 April 2016 [Internet] [updated on 14 April 2016]. [accessed on 20 April 2016]. Available at http://www.who.int/csr/don/14-april-2016-mers-saudi-arabia/en/
- 3.Lee J. Better understanding on MERS corona virus outbreak in Korea. J Korean Med Sci. 2015;30:835–836. doi: 10.3346/jkms.2015.30.7.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korea Centers for Disease Control and Prevention Middle East respiratory syndrome coronavirus outbreak in the Republic of Korea, 2015. Osong Public Health Res Perspect. 2015;6:269–278. doi: 10.1016/j.phrp.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oh MD, Choe PG, Oh HS, Park WB, Lee SM, Park J, Lee SK, Song JS, Kim NJ. Middle East respiratory syndrome coronavirus superspreading event involving 81 persons, Korea 2015. J Korean Med Sci. 2015;30:1701–1705. doi: 10.3346/jkms.2015.30.11.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Summary and risk assessment of current situation in Republic of Korea and China: MERS-CoV risk assessment, 19 June 2015 [Internet] [updated on 19 June 2015]. [accessed on 17 September 2015]. Available at http://who.int/csr/disease/coronavirus_infections/risk-assessment-19june2015/en/
- 7.de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, Hoang DM, Chau NV, Khanh TH, Dong VC, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Zhang A, Wan Y, Liu X, Qiu C, Xi X, Ren Y, Wang J, Dong Y, Bao M, et al. Early hypercytokinemia is associated with interferon-induced transmembrane protein-3 dysfunction and predictive of fatal H7N9 infection. Proc Natl Acad Sci USA. 2014;111:769–774. doi: 10.1073/pnas.1321748111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong CK, Lam CW, Wu AK, Ip WK, Lee NL, Chan IH, Lit LC, Hui DS, Chan MH, Chung SS, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oh MD, Park WB, Choe PG, Choi SJ, Kim JI, Chae J, Park SS, Kim EC, Oh HS, Kim EJ, et al. Viral load kinetics of MERS coronavirus infection. N Engl J Med. 2016 doi: 10.1056/NEJMc1511695. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 11.Park WB, Perera RA, Choe PG, Lau EH, Choi SJ, Chun JY, Oh HS, Song KH, Bang JH, Kim ES, et al. Kinetics of serologic responses to MERS coronavirus infection in humans, South Korea. Emerg Infect Dis. 2015;21:2186–2189. doi: 10.3201/eid2112.151421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Laboratory testing for Middle East respiratory syndrome coronavirus: interim guidance [Internet] [updated on June 2015]. [accessed on17 September 2015]. Available at http://www.who.int/csr/disease/coronavirus_infections/mers-laboratory-testing/en/
- 13.Wang WK, Chen SY, Liu IJ, Kao CL, Chen HL, Chiang BL, Wang JT, Sheng WH, Hsueh PR, Yang CF, et al. Temporal relationship of viral load, ribavirin, interleukin (IL)-6, IL-8, and clinical progression in patients with severe acute respiratory syndrome. Clin Infect Dis. 2004;39:1071–1075. doi: 10.1086/423808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, Al-Rabiah FA, Al-Hajjar S, Al-Barrak A, Flemban H, Al-Nassir WN, Balkhy HH, Al-Hakeem RF, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Assiri A, McGeer A, Perl TM, Price CS, Al Rabeeah AA, Cummings DA, Alabdullatif ZN, Assad M, Almulhim A, Makhdoom H, et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, Law KI, Tang BS, Hon TY, Chan CS, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saad M, Omrani AS, Baig K, Bahloul A, Elzein F, Matin MA, Selim MA, Al Mutairi M, Al Nakhli D, Al Aidaroos AY, et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–306. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Tawfiq JA, Hinedi K, Ghandour J, Khairalla H, Musleh S, Ujayli A, Memish ZA. Middle East respiratory syndrome coronavirus: a case-control study of hospitalized patients. Clin Infect Dis. 2014;59:160–165. doi: 10.1093/cid/ciu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arabi YM, Arifi AA, Balkhy HH, Najm H, Aldawood AS, Ghabashi A, Hawa H, Alothman A, Khaldi A, Al Raiy B. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160:389–397. doi: 10.7326/M13-2486. [DOI] [PubMed] [Google Scholar]
- 20.Menachery VD, Eisfeld AJ, Schäfer A, Josset L, Sims AC, Proll S, Fan S, Li C, Neumann G, Tilton SC, et al. Pathogenic influenza viruses and coronaviruses utilize similar and contrasting approaches to control interferon-stimulated gene responses. MBio. 2014;5:e01174–14. doi: 10.1128/mBio.01174-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faure E, Poissy J, Goffard A, Fournier C, Kipnis E, Titecat M, Bortolotti P, Martinez L, Dubucquoi S, Dessein R, et al. Distinct immune response in two MERS-CoV-infected patients: can we go from bench to bedside? PLoS One. 2014;9:e88716. doi: 10.1371/journal.pone.0088716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee N, Wong CK, Chan PK, Lun SW, Lui G, Wong B, Hui DS, Lam CW, Cockram CS, Choi KW, et al. Hypercytokinemia and hyperactivation of phospho-p38 mitogen-activated protein kinase in severe human influenza A virus infection. Clin Infect Dis. 2007;45:723–731. doi: 10.1086/520981. [DOI] [PubMed] [Google Scholar]
- 23.Lee N, Wong CK, Chan PK, Chan MC, Wong RY, Lun SW, Ngai KL, Lui GC, Wong BC, Lee SK, et al. Cytokine response patterns in severe pandemic 2009 H1N1 and seasonal influenza among hospitalized adults. PLoS One. 2011;6:e26050. doi: 10.1371/journal.pone.0026050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chi Y, Zhu Y, Wen T, Cui L, Ge Y, Jiao Y, Wu T, Ge A, Ji H, Xu K, et al. Cytokine and chemokine levels in patients infected with the novel avian influenza A (H7N9) virus in China. J Infect Dis. 2013;208:1962–1967. doi: 10.1093/infdis/jit440. [DOI] [PubMed] [Google Scholar]
- 25.Zhou J, Chu H, Li C, Wong BH, Cheng ZS, Poon VK, Sun T, Lau CC, Wong KK, Chan JY, et al. Active replication of Middle East respiratory syndrome coronavirus and aberrant induction of inflammatory cytokines and chemokines in human macrophages: implications for pathogenesis. J Infect Dis. 2014;209:1331–1342. doi: 10.1093/infdis/jit504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Wit E, Rasmussen AL, Falzarano D, Bushmaker T, Feldmann F, Brining DL, Fischer ER, Martellaro C, Okumura A, Chang J, et al. Middle East respiratory syndrome coronavirus (MERS-CoV) causes transient lower respiratory tract infection in rhesus macaques. Proc Natl Acad Sci USA. 2013;110:16598–16603. doi: 10.1073/pnas.1310744110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The assay range of ELISA kit
Serial clinical and laboratory results during four weeks from the onset of illness.
Clinical symptoms over the course of MERS-CoV infection.
Shown are the changes in clinical symptoms (fever, sore throat, rhinorrhea, cough, sputum, and diarrhea) during the course of diseases. Clinical symptoms before admission were assessed retrospectively via interview on admission and clinical symptoms after admission to study hospitals were assessed prospectively until discharge. Upper respiratory symptoms such as rhinorrhea and sore throat were rare during the course of disease.
Comparison of cytokine and chemokine levels between the severe group and the mild group.
Hollow circles mean the values measured after the administration of pegylated IFN-α2a.
Changes of the serum levels of cytokine and chemokine over time after symptom onset in the patient with MERS-CoV infection, excluding the measurement values after administration of pegylated interferon alpha-2a.
Comparison of cytokine and chemokine levels between the severe group and the mild group, excluding the measurement values after administration of pegylated interferon alpha-2a.