Abstract
Darwin’s finches are highly innovative. Recently we recorded for the first time a behavioural innovation in Darwin’s finches outside the foraging context: individuals of four species rubbed leaves of the endemic tree Psidium galapageium on their feathers. We hypothesised that this behaviour serves to repel ectoparasites and tested the repellency of P. galapageium leaf extracts against parasites that negatively affect the fitness of Darwin’s finches, namely mosquitoes and the invasive hematophagous fly Philornis downsi. Mosquitoes transmit pathogens which have recently been introduced by humans and the larvae of the fly suck blood from nestlings and incubating females. Our experimental evidence demonstrates that P. galapageium leaf extracts repel both mosquitoes and adult P. downsi and also inhibit the growth of P. downsi larvae. It is therefore possible that finches use this plant to repel ectopoarasites.
Darwin’s finches are highly adaptable and able to cope with the unpredictable characteristics of the Galápagos Archipelago. They show an extraordinary number and diversity of feeding innovations, including consumption of novel food types and foraging behaviours (reviewed in ref. 1). These feeding innovations are novel in the sense that such behaviour patterns are unusual for passerines and it is likely that they evolved after their ancestors colonised the islands. It has been proposed that the ability to innovate so readily has contributed to the adaptive radiation of this clade1. Since the permanent colonisation of the Islands by humans, Darwin’s finches have been exposed to a new set of risks and challenges, in particular exotic parasites and pathogens that reduce the survival and reproductive success of the birds2,3,4,5. Here we report for the first time an innovation of this species group that is outside the feeding context: in 2012 we observed a warbler finch (Certhidea olivacea) tearing off the tips of leaves of the endemic Guayabillo tree, Psidium galapageium, and rubbing them on its feathers. Over the last four years, we have observed ten incidents of this behaviour in four species of Darwin’s finches (see Table 1). We have identified two different methods: (1) sponge method, the bird threads a piece of leaf through its feathers and (2) lotion method, the bird chews the leaf first and applies the mashed leaf to its feathers. In all instances, the birds used P. galapageium leaves, although this tree species occurs at low densities in the highlands of Santa Cruz Island where the observations were made.
Table 1. Observations of topical application of P. galapageium leaves by different Darwin’s finch species.
| Species | Year | Method1 |
|---|---|---|
| Warbler finch (Certhidea olivacea) | 2012 | Sponge |
| Warbler finch (Certhidea olivacea) | 2012 | Lotion |
| Warbler finch (Certhidea olivacea) | 2012 | Sponge |
| Small tree finch (Camarhynchus parvulus) | 2012 | Lotion |
| Small tree finch (Camarhynchus parvulus) | 2012 | Lotion |
| Medium ground finch (Geospizia fortis) | 2014 | Sponge and lotion |
| Small ground finch (Geospizia fuliginosa) | 2014 | Sponge and lotion |
| Small ground finch (Geospizia fuliginosa) | 2014 | Sponge and lotion |
| Warbler finch (Certhidea olivacea) | 2015 | Lotion |
| Small tree finch (Camarhynchus parvulus) | 2015 | Sponge |
1Sponge method: bird threads piece of leaf through the feathers, lotion method: bird chews leave first and applies resulting mixture of saliva and mashed leaf.
Topical application is taxonomically widespread in birds but observations are rare and anecdotal6,7,8,9. Birds have mostly been observed applying arthropods, predominately ants, to their feathers and thus this behaviour is called “active anting”. Reports of birds applying plants to their feathers are rare; only a few instances have been recorded10,11,12. Suggested functions of such behaviour include control of ectoparasites, fungal or bacterial infections12,13,14,15 (but see ref. 16), and/or soothing the skin during moulting7. All observations of the application of P. galapageium leaves were made during the breeding season, when Darwin’s finches do not moult17 thus, soothing is unlikely to be the primary function of this behaviour during this period. We hypothesised that Darwin’s finches use the leaves of P. galapageium to repel ectoparasites and tested the repellent effect of these leaves against parasitic insects that reduce the fitness of Darwin’s finches, namely mosquitoes and the recently introduced parasitic fly Philornis downsi. Several mosquito species are native to the Galápagos but others have been introduced (e.g., Culex quinquefasciatus, Aedes aegypti18). More importantly, the appearance in the Galápagos of novel mosquito-borne pathogens such as avian pox is increasingly affecting the survival of Darwin’s finches19.
The reproductive success of Darwin’s finches is strongly reduced by the larval stage of P. downsi, a recently introduced, obligate bird parasite (reviewed in refs 20,21). The first larval stage is mainly found in the chicks’ nostrils. The second and third larval stages live in the bottom of the nest, pierce the skin of the chicks, and consume their blood22. However, for the first time in 2012 and again in 2014 and 2015 we recorded P. downsi larvae in nests that had been abandoned during incubation and in which no chicks had ever been present. As Darwin’s finches never re-use nests (personal observation) the larvae could not have hatched during a previous breeding attempt. Previously larvae had been found only in nests with hatchlings and never in nests containing unhatched eggs3,21,23. A total of 21 nests which had been abandoned during incubation were examined between 1998 and 2010. As this sample size is small it is possible that previous studies overlooked larvae in incubating nests. However, infestation during the incubation phase could also represent a gradual change of the parasite’s life cycle. In 2012 pupae and larvae of all developmental stages were found in 88% of abandoned warbler finch nests (15 out of 17 nests) and in 22% of abandoned small tree finch nests (2 out of 9 nests, numbers of P. downsi specimens ranged from 1 to 13, median 2). The only available blood source in such nests is the incubating female suggesting that they are also being parasitized by P. downsi larvae. Independent support for this comes from the finding that incubating female medium ground finches from parasitized nests showed higher P. downsi-specific antibody levels than females from parasite free nests24. Based on this knowledge about the negative effect of mosquitoes and P. downsi on Darwin’s finches we hypothesized that the behaviour of rubbing plants into feathers could have one or more possible effects: the plant extract might deter mosquitoes from biting; it might prevent adult P. downsi flies from entering the nest; it might protect incubating females from being bitten by P. downsi larvae or it might affect larval growth. In the light of these possible effects, we tested the repellent effect of P. galapageium on mosquitoes, on adult P. downsi flies and also its effect on the growth of P. downsi larvae.
Results
Repellent effect of P. galapageium on Mosquitoes
Field-experiment
We tested the repellent effect of P. galapageium on the local mosquito fauna in a field experiment using 17 human subjects, each of whom treated one leg and one arm with crushed P. galapageium leaves and left the other arm and leg untreated. During a 15-mintutes-exposure to mosquitoes in the field a total of 202 mosquito bites were counted. Significantly fewer mosquitoes than expected by random bit the limbs treated with P. galapageium (mean proportion of bites on treated limb ± standard error: 0.27 ± 0.11, Chi-squared Test for equal or given proportions, χ2 = 48.04, n = 17, p < 0.001)
Lab-experiment
In the laboratory, we assessed the response of the mosquito Anopheles arabiensis to blood-filled sausages treated with an ethanol extract of P. galapageium leaves compared to two controls, one treated with ethanol and the second with an extract of Rubus idaeus. Significantly fewer mosquitoes than expected by random landed on the sausage treated with P. galapageium extract than on the ethanol-treated control sausage (mean proportion of mosquitos on ethanol-treated sausage ± standard error: 0.99 ± 0.03, Chi-squared Test for equal or given proportions, χ2 = 138.99, n = 10, p < 0.0001, Fig. 1, Table S2). The mosquitoes significantly avoided the R. idaeus extract when tested against the sausage treated with ethanol (mean proportion of mosquitos on ethanol-treated sausage ± standard error: 0.81 ± 0.12, Chi-squared Test for equal or given proportions, χ2 = 56.437, n = 10, p < 0.0001, Fig. 1, Table S2). However, significantly more mosquitoes landed on the sausage treated with R. idaeus when tested against the sausage treated with P. galapageium (mean proportion of mosquitos on R. idaeus-treated sausage ± standard error: 0.91 ± 0.09, Chi-squared Test for equal or given proportions, χ2 = 60.59, n = 10, p < 0.0001, Fig. 1, Table S2).
Figure 1. Repellent effect of P. galapageium on Anopheles arabiensis.
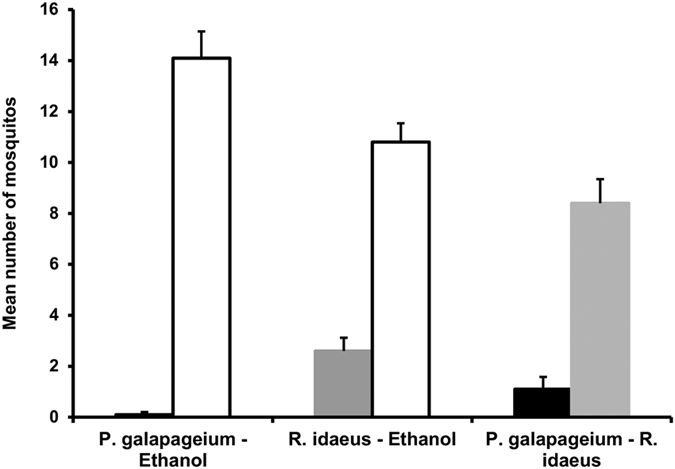
Mean number (+SE) of mosquitoes which landed on the sausage treated with P. galapageium (black bar, only one mosquito landed in total) versus ethanol (white bar), R. idaeus (grey bar) versus ethanol (white bar) and which landed on the sausage treated with P. galapageium (black bar) versus R. idaeus extract (grey bar).
Effect of P. galapageium on Philornis downsi
Effect on larval growth
We also measured the effect of P. galapageium leaves on larval growth (measured as weight after two days) compared to the two control treatments (water and Tradescantia flumensis, a plant which is not known to have a repellent effect) and included the initial weight of the larva as a co-variable in the model (linear model: F = 25.41, df = 68, p < 0.001 and adjusted R2 = 0.51). Larvae that fed on chicken blood through gauze treated with P. galapageium leaves had significantly lower weight gain compared to the weight gain of larvae in two control groups (Post-hoc comparison of group means adjusted for the covariate initial weight: Tukey’s honest significant difference (HSD) test, P. galapageium – Water, T60 = 2.83, p = 0.02; P. galapageium – T. fluminensis, T31 = 2.59, p = 0.02, Fig. 2).
Figure 2. Effect of P. galapageium on larval growth of P. downsi.
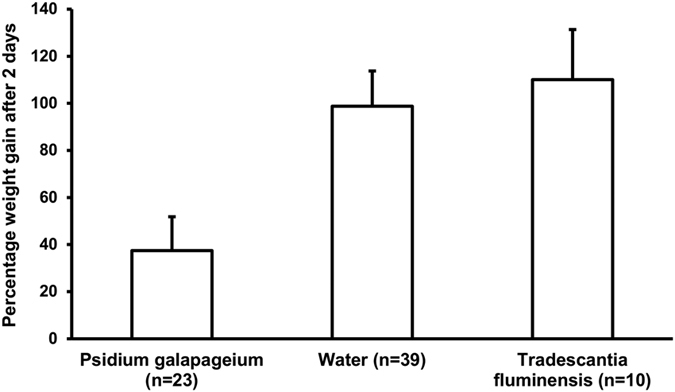
Mean (+SE) percentage of weight gain after two days of P. downsi larvae which fed from a blood source treated with mashed P. galapageium leaves, water or mashed Tradescantia fluminensis leaves, a non-repellent plant. Percentage weight gain was calculated as (“Weight after 2 days” − “Initial weight”)/“Initial weight” × 100.
Effect on adult flies
To test whether P. galapageium repels adults of P. downsi we used a Y-tube laboratory olfactometer. The flies could choose between entering the arm which delivered the odour of activated yeast, which is attractive to this species (see methods), and the other arm which delivered a combined odour of activated yeast and P. galapageium extract. Of 37 females tested, 18 chose the control arm (activated yeast only), five chose the P. galapageium treatment (activated yeast combined with P. galapageium extract) and 14 did not respond. The 23 flies that responded chose the control treatment significantly above chance (binomial test: p = 0.011).
Coupled Gas Chromatographic-Electroantennographic Detection (GC-EAD)
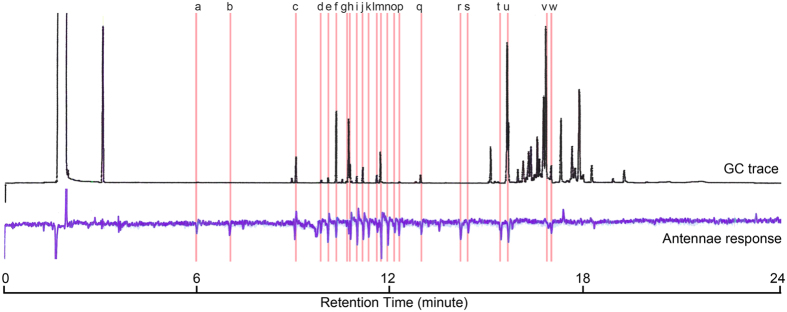
We conducted GC-EAD analysis to verify that the olfactory system of P. downsi includes receptors for volatile components of P. galapageium leaves. Philornis downsi antennae responded to 23 compounds in the P. galapageium extracts (Fig. 3), which are listed in Table 2 with their respective retention indices. The antennally active compounds are principally monoterpenoids and sesquiterpenes.
Figure 3. Representative GC-EAD analysis that tested the antennae of adult female P. downsi (lower trace) for responses to volatile components of P. galapageium leaf extracts (upper trace).
Twenty-three compounds (indicated by vertical lines) consistently elicited responses from P. downsi antennae. Letters of the 23 antennally active compounds correspond to those in Table 2.
Table 2. P. galapageium leaf compounds eliciting GC-EAD responses from P. downsi.
| Chemicals | Retention index | |
|---|---|---|
| a | 3-Hexenal | |
| b | Diacetone alcohol | 845 |
| c | α-Pinene | 936 |
| d | β-Pinene | 980 |
| e | β-Myrcene | 993 |
| f | α-Phellandrene | 1007 |
| g | D-Limonene | 1034 |
| h | Eucalyptol | 1036 |
| i | β-Ocimene | 1051 |
| j | γ-Terpinene | 1064 |
| k | Guaiacol | 1072 |
| l | α-Terpinolen | 1093 |
| m | Linalool | 1101 |
| n | unknown | 1112 |
| o | unknown | 1128 |
| p | unknown | 1146 |
| q | α-Terpineol | 1197 |
| r | unknown | 1296 |
| s | unknown | 1318 |
| t | Eremophilene | 1413 |
| u | β-Caryophyllene | 1442 |
| v | (E)-Nerolidol | 1572 |
| w | unknown | 1584 |
Discussion
Our experiments suggest the repellent effect of P. galapageium on mosquitoes and adult P. downsi and show a growth inhibiting effect on P. downsi larvae. We recognize that the actual concentration of the plant volatiles applied by a bird to its feathers can be different than the concentration tested in our behavioural assays. In Y-tube bioassays, flies were behaviourally sensitive to one tenth equivalent amount of the leaf extract, suggesting that some of the tested leaf volatiles could maintain repellency at reasonably low concentrations. It is also possible that the human skin microbiome or the alcohol used in the blood sausage assays could have affected the perception of the mosquitoes. However, we were able to demonstrate that P. galapageium has a repellent effect using three different extraction methods (crushed leaves, alcohol extraction, dichloromethane extraction). The fact that consistent results were obtained with each method supports the conclusion that P. galapageium is repellent under a variety of background conditions. These findings are in line with our hypothesis that the behaviour of self-treating feathers with P. galapageium leaves confers a degree of protection from parasitism. These lab based experiments are good indirect evidence for this effect but further studies are needed to confirm that this behaviour does indeed provide this form of protection for Darwin’s finches.
Preening with P. galapageium may be considered an example of self-medication, which is defined as defence against parasites, pathogens, or both, by one species applying substances produced by another species25. Clayton and Wolf 25 suggest three aspects that characterise self-medication. Here we provide evidence for two aspects: (1) that the medical substance is deliberately contacted by the medicator and (2) that the substance has a negative effect on the parasite25. However, de Roode et al.26 point out that self-medication may not necessarily lead to a reduction of parasites. It could also enhance host fitness by increasing tolerance of infection. The third aspect suggested by both Clayton and Wolf 25 and de Roode et al.26 is, that the behaviour increases the fitness of the medicator. The repellent quality of P. galapageium in situ and evidence for increased fitness of the medicator as a result of using the substance remain to be tested.
Self-medication in the form of ingestion of medicinal plants is widespread in great apes (reviewed in ref. 27) but also in insects. For instance moths increase the ingestion of particular chemicals that are already in their diets28. Most examples of medicinal plant ingestion are forms of therapeutic self-medication in which diseased individuals change their behaviour in response to parasitic infection. In contrast, prophylactic self-medication is used by infected and uninfected individuals to prevent parasite infection often in response to high parasite risk28,29. For instance, several bird species (reviewed in ref. 30) and wood ants Formica paralugubris29 have been observed incorporating plant material with medicinal properties into their nests.
Topical application is a subcategory of self-medication which is rare (reviewed in ref. 25). While there are only few studies that report insecticidal effects of substances applied by birds which lead to a reduction of their ectoparasite load (reviewed in refs 12 and 25), we have shown that P. galapageium has a repellent rather than an insecticidal effect on adult P. downsi and on mosquitoes. Preening with this plant may therefore, prevent the parasites making contact with the birds in the first place rather than eliminating the insects after potentially detrimental contact has been made. Thus, it may represent a form of prophylactic self-medication. The effect on the larvae of P. downsi may be insecticidal or repellent. Ingestion of P. galapageium on the gauze could have an insecticidal effect and/or the larvae were repelled by the plant and did not ingest as much food as the control groups. Our current assay does not allow us to distinguish between these two possibilities.
Many plants produce secondary metabolites to protect against herbivores and pathogens. Several volatile components of P. galapageium that we have demonstrated that P. downsi can detect also occur in other plant species, which have been shown to have repellent effects on arthropods. For instance Psidium guajava produces volatiles that repel the sapsucking insect Diaphorina citri31,32,33. The volatile constituents of P. guajava that have repellent properties are the three part combination of limonene, α-pinene and β-pinene33. All three of these compounds are present in P. galapageium and elicited antennal responses in our GC-EAD analysis. Other components that induced antennal responses in our analysis include β-caryopyllene and linalool. β-Caryopyllene is one of the dominant components of P. galapageium and has been shown to have a repellent effect on mosquitoes of the genera Armigeres, Culex and Aedes34. Linalool has been found to act as a repellent against several species of mosquitoes of the genera Aedes and Culex35,36,37. It remains to be determined precisely which volatiles of P. galapageium repel P. downsi and local mosquito species, but as we have shown, P. galapageium contains multiple components that have been demonstrated to have repellent effects on a wide variety of arthropods. This suggests that the exploitation of this plant’s secondary metabolites by finches is effective.
In sensitive ecosystems such as those of the Galápagos Islands the use of insecticides is problematic due to effects on non-target organisms38 including natural enemies39, beneficial organisms40 and wildlife in general41,42. Thus, the discovery of the repellent effect of an endemic plant is potentially important for the conservation of threatened or endangered endemic bird populations suffering from non-native parasites and pathogens. Several studies have successfully reduced the number of P. downsi larvae in Darwin’s finch nests by using applied insecticides (e.g. refs 24,43 and 44). However, they all used substances that are potentially toxic to non-target organisms. Although numerous plant compounds are known for their repellent effects45,46 (and see above) the number of commercially available bio-insecticides is surprisingly low. Thus, the identification of the active compounds of P. galapageium might serve as basis for developing a novel plant-based repellent.
The behaviour reported in this paper is yet another example of exceptional behavioural flexibility and innovation in Darwin’s finches. Several definitions of innovation have been proposed (reviewed in ref. 47). According to Kummer and Goodall48 innovations can be an existing solution to a novel problem or a novel solution to an existing problem and can be based on already existing, completely new or modified behaviour patterns. In the current example, preening is an already existing behaviour and the novel aspect is the incorporation of an object into the behavioural sequence. It can be seen as a form of active anting, i.e. grabbing ants or other objects and rubbing them into the feathers. According to recent definitions, active anting is now accepted as a form of tool use49,50,51,52. Anting is taxonomically widespread in birds and thus might be an ancestral trait in Darwin’s finches but the use of P. galapageium for this purpose evolved after their ancestors colonised the islands.
We can only speculate how the use of P. galapageium leaves became incorporated into the sequence of preening. Increasing evidence that passerines have sufficient olfactory abilities to detect chemical cues (reviewed in ref. 53) raises the possibility that such cues could be used to select appropriate objects for anting15. Clark and Mason54,55 showed that starlings and brown-headed cowbirds could be conditioned to discriminate between different plant volatiles. The proximate mechanism by which preening with plants is initially reinforced might be that it either reduces disturbance by insects and/or reduces the itching sensation caused by insect bites. Whether P. galapageium has such properties still needs to be tested.
There is no direct evidence to indicate when the use of P. galapageium leaves emerged in Darwin’s finches. It is possible that this form of tool-use may have existed for a long time and has gone unnoticed despite the fact that several research groups have studied the behaviour of Darwin’s tree finches at the same study site for two decades. It is also possible that an existing behaviour has increased in frequency during recent years in response to the selection pressure exerted by novel mosquito-borne pathogens and the recently introduced parasite P. downsi.
Methods
Field Observations
Observations were made opportunistically during a field study on the influence of Philornis downsi on the breeding success of Darwin’s finches. This study was conducted at a study site (Los Gemelos, 0°37’34” S, 90°23’10” W) in the humid “Scalesia” forest on the Island of Santa Cruz, between January 11 - April 25 2012, January 9 - April 31 2014 and January 15 - May 30 2015. During this period P. downsi prevalence was very high. In 2012 all investigated nests with chicks of warbler finches (n = 46) and small tree finches (n = 37) were infested by P. downsi larvae. In 2014 and 2015 the prevalence was similar: 2014 warbler finch 97.9% (n = 96), small tree finch 95.8% (n = 72); 2015 warbler finch 94.1% (n = 51), small tree finch 90.2% (n = 51). However, we have no data on the prevalence of mosquitoes in the study area.
Repellent effect of P. galapageium on Mosquitoes
Field-experiment
In the field, the 17 human subjects (10 men, 7 women between 21 and 56 years old, see Table S1) treated one leg and one arm with ten crushed P. galapageium leaves each and left the other arm and leg untreated. The side of treatment was assigned randomly. Participants were standing while the experiment. The number of mosquitoes which were observed biting within 15 min of exposure on treated and non-treated limbs was counted. The experiment was carried out on the 9th of March 2016 at 6:33 pm (participants 1–9) and on the 18th of March 2016 at 6:30 pm (participants 10–17) at the Charles Darwin Station, Puerto Ayora, Galápagos. All participants were given the instruction not to shower two hours previous to testing and not to use repellents. To test whether the choice of the mosquitoes was different from random we used a “test of equal or given proportions” prob.test. library stats, R 3.2.2.,)56. Sample size was defined by the number of treated subjects (17), which were tested only once to avoid pseudoreplication.
Lab-experiment
The experiment was conducted at the Insect Pest Control Laboratory (IPCL) of the Joint FAO/IAEA Division, Seibersdorf, Austria. The Anopheles arabiensis used in this study originated from Dongola in the northern state of Sudan in 2005 and have been maintained and reared since then in the laboratory (for details see ref. 56). Adults were kept in a climate-controlled room maintained at a temperature of 27 ± 1 °C and 60 ± 10% relative humidity on a 12:12 h photoperiod, including dusk (1 h) and dawn (1 h). For the experiments, 20 five-day old female An. arabiensis were placed in a 30 cm cubic insect cage (Megaview Science Education Services Co., Ltd., Taiwan) the day before the experiment started and fed with 5% sugar-solution.
To test the repellent effect of P. galapageium, collagen sausage casings (Edicas 23NC, FIBRAN S.A., Girona, Spain) filled with 50 ml of defibrinated bovine blood heated at 38–39 °C were used. Each sausage was moistened with extract of P. galapageium or control plant or with their respective solvent. To prepare the P. galapageium extract, dried and ground P. galapageium leaves, collected at Los Gemelos, Santa Cruz, Galápagos, were put in 70% ethanol for one week at a weight ratio of 1:10 ground leaves to ethanol. After seven days the extract was filtered and preserved in a sealed glass bottle at room temperature until use.
During the experiment, two sausages were simultaneously presented to the female mosquitoes. One sausage was moistened with repellent while the second sausage was moistened with the control plant or solvent. The sausages were placed in the cage in parallel separated by 10 cm and the experimenter blew 5 times into the cage to motivate the mosquitoes to move. The following combination was tested: P. galapageium extract against ethanol. To test whether the application of a plant extract has a repellent effect per se, we introduced an additional control using Rubus idaeus as a plant species that is not known to have repellent properties and is closely related to the invasive Rubus niveus which was very abundant in our study area. We prepared the R. ideaeus extract in the same way as the P. galapageium extract and tested it against the solvent ethanol. Contrary to our expectation R. ideaus had a repellent effect compared to ethanol. In a subsequent experiment we thus tested P. galapageium extract against R. idaeus extract to assess which of the two plants had the stronger repellent effect. To measure the repellent effect we counted the number of mosquitoes sitting on each sausage after 60 seconds of exposure and repeated this every minute, for nine more minutes. The results presented are based on the number of mosquitoes, which landed on the treated sausage (P. galapageium, R.ideaus) after the first 60 seconds of exposure to the sausages. However, in the subsequent 9 min the percentage of mosquitoes that landed on the sausage treated with P. galapageium was similar to the results from the first 60 seconds (first 60 sec: median 3.3%, range 0–41.5%; subsequent 9 min.: median 0%, range 0–45%).
We conducted ten replicates for each condition. For each replicate new sausages and new cages with different mosquitoes were used. The side of treatment and control was assigned randomly. The number of female mosquitoes, which were still alive when the experiment started, ranged from 15 to 20 (median 19) in each trial. During the first 60 seconds the percentage of mosquitoes which landed on either of the sausages in all trials and conditions ranged from 29–100% (median 66%). All data are shown in Table S2. Again we used a test of equal or given proportion to estimate whether the choice of the mosquitoes in each comparison (P.galapageium – ethanol, R. idaeus – ethanol, P. galapageium – R. idaeus) was different from random (prob.test. library stats, R 3.2.2.57). We have adjusted the significance level to p = 0.017 using a Bonferroni correction
Repellent effect of P. galapageium on P. downsi
Effect on larval growth
In a laboratory experiment, we tested whether P. galapageium leaves reduce larval growth of P. downsi. Larvae were collected from the study site “Los Gemelos” between 07th February and 1st April 2014, which is the main breeding season of Darwin’s finches (Table S3). We only used second instar, small to medium sized larvae from two different size classes: <0.5 cm small larvae, 0.5 cm–1.0 cm medium larvae. Larvae were assigned randomly to test and control group and raised in the laboratory at the Charles Darwin Station, Santa Cruz, Galápagos, and fed with chicken blood that was applied to a piece of cotton, which was tamped into a short piece of plastic drinking straw. The tip of the straw was covered with gauze. One group had to feed through gauze on which one drop of crushed P. galapageium leaves was applied. To produce the crushed leaves, four leaves and 1 ml of water were put into an Eppendorf tube (2 ml) and crushed with metal forceps. The other group had to feed through gauze that was treated with water only. Blood and treatment was renewed daily. To test whether the application of a plant extract had a repellent effect per se, we introduced an additional control group with crushed Tradescantia fluminensis leaves, a plant species present on the Galápagos Islands that is not known to have repellent properties. Biosafety regulations severely limit the movement of plants into and out of the Galápagos, therefore we used a different control plant in this lab experiment which was conducted in the Galápagos than that used in the lab-experiment on the repellent effect of P. galapageium on mosquitoes described above which was conducted in Austria. We weighed the larvae before the experiment and after 2 days of treatment and calculated the percentage of weight gain as “weight after 2 days” − “initial weight”)/“initial weight” × 100. Raw data are presented in Table S3. To analyse the effect of P. galapageium leaves on larval growth compared to the two control treatments (water and T. fluminensis) we calculated a linear model with weight after 2 days as predicted variable and treatment and initial weight as predictor variable. We calculated the model with interactions, but since none was significant we excluded them from the final model. We omitted one larva of the P. galapageium treatment group as its weight decreased by the power of ten, likely representing a typing error in data entering. However, the results did not change without this outlier. Weights were log-transformed. The post-hoc tests were done with the R-package multcomp58.
Effect on adult flies
We used a Y-tube laboratory olfactometer to assess the repellent effect of P. galapageium extract on the attractiveness of activated yeast to P. downsi. Olfactometer arms were 30 cm × 6 cm dia., and the angle between the arms was 70°. The y-tube was positioned vertically and a fluorescent lamp with dual bulbs (1.2 m) was centred 50 cm above the olfactometer parallel to a line connecting the ends of the olfactometer arms. Charcoal filtered air was introduced (1.0 L/min) into Erlenmeyer flasks (125 mL) fitted with two-hole rubber stoppers for incurrent and excurrent air flows. Effluent from the flasks was transferred to the olfactometer arms through plastic tubing (5 mm i.d.) and connected to a glass adapter with a barbed fitting on one end and a ground glass fitting (6 cm dia.) on the other end. The positions of the control and treatment were switched after every five trials. Adult P. downsi were released in the centre arm of the olfactometer and allowed to fly upwards towards the light and odour sources. A positive or negative response was recorded if the fly stayed in the distal half of the treatment or control arm for at least one minute. The majority of responding flies chose one arm within ten seconds.
The first experiment was intended to establish the attractiveness of yeast and the veracity of the olfactometer assay. The test stimulus was activated yeast (0.5 g baker’s yeast, 2.5 g sugar, 30 mL water) and the control was deionized water (30 mL). Of the 38 assays of P. downsi males 13 chose the yeast treatment, 9 chose the water control and 16 were unresponsive. The 22 responsive males showed no significant preference for the yeast treatment (binomial test: p = 0.12). Of the 60 assays of P. downsi females 25 chose the yeast treatment, 11 chose the water control and 24 were unresponsive. The 36 responsive females showed a significant preference for the yeast treatment (binomial test: p = 0.01). Thus, only female flies were used for the following experiment.
The purpose of the second experiment was to determine the repellent effect of P. galapageium extracts. Leaves of P. galapageium were collected in the Los Gemelos highlands of Santa Cruz Island on 26 February, 2015, transported to the Charles Darwin Research Station, Puerto Ayora, and stored at 4 °C. Leaf extracts were made within 24 hours by placing four crushed P. galapageium leaves in a vial (4 mL) with dichloromethane (4 mL) for 10 minutes and then decanting the solvent extract into a fresh vial. Thus, each assay used 0.1 P. galapageium leaf equivalents (4 leaves in 4 mL DCM = a concentration of 1.0 leaf/mL; the 100 uL aliquots used in the assays would represent 0.1 leaf eq.). It is impossible to say how much volatile material a finch applies to its feathers, but based on our field observations, a tenth of one leaf is reasonably close to the amount used by finches. In Syracuse, extracts were stored at −60 °C until use. An aliquot (100 μL) of the extract was placed on a filter paper strip inside a glass tube that was inserted in the plastic tubing between the Erlenmeyer flask and the olfactometer arm.
Controls consisted of an equal volume of methylene chloride on a filter paper strip. The Erlenmeyer flasks of both the treatment and control contained activated yeast as described above.
Coupled Gas Chromatographic-Electroantennographic Detection (GC-EAD)
In a further experiment coupled Gas Chromatographic-Electroantennographic Detection was carried out to give insight into which chemical compounds of P. galapageium leaves can be detected by adult P. downsi. Coupled GC-EAD analysis was performed using a gas chromatograph (Hewlett-Packard 5890 Series II) equipped with a capillary column (HP5-MS, 30 m × 0.25 mm ID, 0.25 μm film thickness; Agilent Technologies, Wilmington, DE, USA) in splitless mode with 1 min sampling. The oven temperature was programmed for 5 min at 40 °C, then increased at 15 °C/min to 250 °C, and then held for 5 min. The injector temperature was 250 °C. Helium was the carrier gas at a flow rate of 1 mL/min. The column effluent was split 1:1 in the oven via a glass Y-connector with nitrogen make-up gas (8 mL/min) introduced through a second glass Y-connector. One arm of the splitter led to the flame ionization detector (FID) (260 °C) and the other to a heated transfer line (260 °C) (Agilent Technologies, Wilmington, DE, USA). The EAD effluent was introduced into a cooled (5 °C) humidified air stream (1 L/min) directed toward the antennae of the mounted fly.
Whole head preparations were made of individual flies, age 3–6 d, for GC-EAD analysis, as described previously from similar studies with other flies59,60,61. The head was excised and the antennae were positioned between two gold-saline (Drosophilaringer solution; 46 mmol NaCl, 182 mmol KCl, 3 mmol CaCl2, and 10 mmol Tris HCl at pH 7.2) electrode micropipettes in an acrylic holder. The output signal from the antenna was amplified (10×) by a custom high input impedance DC amplifier and recorded on an integrator-recorder.
For the GC-EAD analysis of P. galapageium leaf extracts, a total of four antennal pairs from two female and two male flies (1–2 replicated runs/pair) were tested.
To identify the chemical compounds present in the P. galapageium leaf extract to which P. downsi antennae were responding, leaf extracts were analysed by coupled gas chromatography-mass spectrometry (GC-MS; Agilent 7890A GC interfaced to a 5975 mass selective detector in EI mode, 70 eV; Agilent Technologies, Santa Clara, CA, USA). The GC column, temperature program and carrier gas were the same as those used for GC-EAD. Compound identifications were based on matches with spectra in the NIST/EPA/NIH Mass Spectral Library Version 2.0f (2009) and retention index (Table 2).
Ethical Statement
The study was conducted in the protected areas of the Galápagos National Park. Permission to conduct this study was granted by the Galápagos National Park and the Charles Darwin research station (Project: PC-54-11 and Project: PC-02-14; Permit Nr. PR.CDS.ACI.P01.R02). For the experiment involving human subjects all experimental protocols were approved by the Charles Darwin research station (FCD-DEJ-16-167), and informed consent was obtained from all subjects. All methods were carried out in accordance with the approved guidelines.
Additional Information
How to cite this article: Cimadom, A. et al. Darwin’s finches treat their feathers with a natural repellent. Sci. Rep. 6, 34559; doi: 10.1038/srep34559 (2016).
Supplementary Material
Acknowledgments
We thank Denis A. Mosquera Muñoz, David Anchundia, Janis Thailayil and Chrisitian Wappl for field assistance and help conducting the experiments. We are grateful to Caroline Raby for improving the English and comments on the manuscript and to Barbara Fischer for statistical advise. The study was partly funded by the Galápagos Conservation Trust, the Ethologische Gesellschaft e.V., the FWF (project number P 26556-B22 granted to Sabine Tebbich) and the International Community Foundation with a grant awarded by The Leona M. and Harry B. Helmsley Charitable Trust to Stephen Teale, Birgit Fessl and Charlotte Causton (FCD 2140).
Footnotes
Author Contributions A.C., C.C., D.H.C., D.D., R.H.-N., P.L., S.A.T. and S.T. conceived and designed the experiments; A.C., C.C., D.H.C., B.F., A.E.M., E.M.S., S.A.T. and S.T. collected the data and performed the experiments; A.C., C.C., D.H.C., E.M.S., S.A.T. and S.T. analysed the data; D.D., P.L., A.E.M., E.N., R.H.-N. and S.A.T. contributed reagents/materials/analysis tools; A.C., D.H.C., D.D. E.N., S.A.T. and S.T. wrote the paper.
References
- Tebbich S., Sterelny K. & Teschke I. The tale of the finch: adaptive radiation and behavioural flexibility. Philosophical Transactions of the Royal Society B-Biological Sciences 365, 1099–1109, 10.1098/rstb.2009.0291 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedenfeld D. A., Jimenez-Uzcategui G., Fessl B., Kleindorfer S. & Valarezo J. C. Invasive pathogens as threats for island species. Pacific Conservation Biology 13, 14–19 (2007). [Google Scholar]
- Fessl B. & Tebbich S. Philornis downsi - a recently discovered parasite on the Galapagos archipelago - a threat for Darwin’s finches? Ibis 144, 445–451, 10.1046/j.1474-919X.2002.00076.x (2002). [DOI] [Google Scholar]
- Wikelski M., Foufopoulos J., Vargas H. & Snell H. Galapagos birds and diseases: Invasive pathogens as threats for island species. Ecology and Society 9 (2004). [Google Scholar]
- Causton C. E. et al. Alien insects: Threats and implications for conservation of Galapagos Islands. Annals of the Entomological Society of America 99, 121–143, 10.1603/0013-8746(2006)099[0121:aitaif]2.0.co;2 (2006). [DOI] [Google Scholar]
- Craig A. Anting in Afrotropical birds: a review. Ostrich 70, 203–207, 10.1080/00306525.1999.9634237 (1999). [DOI] [Google Scholar]
- Potter E. F. Anting in wild birds, its frequency and probable purpose. Auk 87, 692-& (1970). [Google Scholar]
- Simmons K. E. L. A review of the anting-behaviour of passerine birds. British Birds 50, 401–424 (1957). [Google Scholar]
- Wenny D. Three-striped Warbler (Basileuterus tristriatus) “anting” with a caterpillar. Wilson Bulletin 110, 128–131 (1998). [Google Scholar]
- Nero R. W. & Hatch D. R. M. Common Grackles anting with marigold flowers. Blue Jay 42, 212–214 (1984). [Google Scholar]
- VanderWerf E. A. ‘Elepaio “anting” with a garlic snail and a Schinus fruit. Journal of Field Ornithology 76, 134–137 (2005). [Google Scholar]
- Weldon P. J. et al. Anointing Chemicals and Hematophagous Arthropods: Responses by Ticks and Mosquitoes to Citrus (Rutaceae) Peel Exudates and Monoterpene Components. Journal of Chemical Ecology 37, 348–359, 10.1007/s10886-011-9922-7 (2011). [DOI] [PubMed] [Google Scholar]
- Dennis J. Commentary on grackles anting with marigold blossoms. Blue Jay 43, 175–177 (1985). [Google Scholar]
- Ehrlich P. R., Dobkin D. S. & Wheye D. The Adaptive Significance of Anting. Auk 103, 835–835 (1986). [Google Scholar]
- Clark C. C. & Clark L. Anting behavior by common grackles and european starlings. Wilson Bulletin 102, 167–169 (1990). [Google Scholar]
- Revis H. C. & Waller D. A. Bactericidal and fungicidal activity of ant chemicals on feather parasites: An evaluation of anting behavior as a method of self-medication in songbirds. Auk 121, 1262–1268, 10.1642/0004-8038(2004)121[1262:bafaoa]2.0.co;2 (2004). [DOI] [Google Scholar]
- Snow D. W. Moult and the breeding cycle in Darwin’s finches. Journal of Ornithology 107, 283–291 (1966). [Google Scholar]
- Sinclair B. J. In Charles Darwin Foundation Galapagos Species Checklist - Lista de Especies de Galápagos de la Fundación Charles Darwin. Charles Darwin Foundation/Fundación Charles Darwin, Puerto Ayora, Galapagos Vol. Last updated: 11 Feb 2014 (eds Bungratz F. et al.) (http://www.darwinfoundation.org/datazone/checklists/terrestrial-invertebrates/diptera/ 2014). [Google Scholar]
- Parker P. G. et al. 110 Years of Avipoxvirus in the Galapagos Islands. Plos One 6, 10.1371/journal.pone.0015989 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koop J. A. H., Huber S. K., Laverty S. M. & Clayton D. H. Experimental Demonstration of the Fitness Consequences of an Introduced Parasite of Darwin’s Finches. Plos One 6, 10.1371/journal.pone.0019706 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimadom A. et al. Invasive Parasites, Habitat Change and Heavy Rainfall Reduce Breeding Success in Darwin’s Finches. Plos One 9, 10, 10.1371/journal.pone.0107518 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessl B., Sinclair B. J. & Kleindorfer S. The life-cycle of Philornis downsi (Diptera: Muscidae) parasitizing Darwin’s finches and its impacts on nestling survival. Parasitology 133, 739–747, 10.1017/s0031182006001089 (2006). [DOI] [PubMed] [Google Scholar]
- O’Connor J. A., Robertson J. & Kleindorfer S. Video analysis of host-parasite interactions in nests of Darwin’s finches. Oryx 44, 588–594, 10.1017/s0030605310000086 (2010). [DOI] [Google Scholar]
- Koop J. A. H., Owen J. P., Knutie S. A., Aguilar M. A. & Clayton D. H. Experimental demonstration of a parasite-induced immune response in wild birds: Darwin’s finches and introduced nest flies. Ecology and Evolution 3, 2514–2523, 10.1002/ece3.651 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. H. & Wolfe N. D. The adaptive significance of self-medication. Trends in Ecology & Evolution 8, 60–63, 10.1016/0169-5347(93)90160-q (1993). [DOI] [PubMed] [Google Scholar]
- de Roode J. C., Lefevre T. & Hunter M. D. Self-Medication in Animals. Science 340, 150–151, 10.1126/science.1235824 (2013). [DOI] [PubMed] [Google Scholar]
- Huffman M. A. Animal self-medication and ethno-medicine: exploration and exploitation of the medicinal properties of plants. Proceedings of the Nutrition Society 62, 371–381, 10.1079/pns2003257 (2003). [DOI] [PubMed] [Google Scholar]
- Singer M. S., Mace K. C. & Bernays E. A. Self-Medication as Adaptive Plasticity: Increased Ingestion of Plant Toxins by Parasitized Caterpillars. Plos One 4, 8, 10.1371/journal.pone.0004796 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castella G., Chapuisat M. & Christe P. Prophylaxis with resin in wood ants. Animal Behaviour 75, 1591–1596, 10.1016/j.anbehav.2007.10.014 (2008). [DOI] [Google Scholar]
- Gwinner H. & Berger S. European starlings: nestling condition, parasites and green nest material during the breeding season. Journal of Ornithology 146, 365–371, 10.1007/s10336-005-0012-x (2005). [DOI] [Google Scholar]
- Rouseff R. L., Onagbola E. O., Smoot J. M. & Stelinski L. L. Sulfur volatiles in guava (Psidium guajava L.) leaves: Possible defense mechanism. Journal of Agricultural and Food Chemistry 56, 8905–8910, 10.1021/jf801735v (2008). [DOI] [PubMed] [Google Scholar]
- Zaka S. M., Zeng X. N., Holford P. & Beattie G. A. C. Repellent effect of guava leaf volatiles on settlement of adults of citrus psylla, Diaphorina citri Kuwayama, on citrus. Insect Science 17, 39–45, 10.1111/j.1744-7917.2009.01271.x (2010). [DOI] [Google Scholar]
- Zaka S. M., Zeng X. N. & Wang H. T. Chemotaxis of Adults of the Asiatic Citrus Psyllid, Diaphorina citri Kuwayama, to Volatile Terpenes Detected from Guava Leaves. Pakistan Journal of Zoology 47, 153–159 (2015). [Google Scholar]
- Vongsombath C., Palsson K., Bjork L., Borg-Karlson A. K. & Jaenson T. G. T. Mosquito (Diptera: Culicidae) Repellency Field Tests of Essential Oils From Plants Traditionally Used in Laos. Journal of Medical Entomology 49, 1398–1404, 10.1603/me12025 (2012). [DOI] [PubMed] [Google Scholar]
- Kline D. L., Bernier U. R., Posey K. H. & Barnard D. R. Olfactometric evaluation of spatial repellents for Aedes aegypti. Journal of Medical Entomology 40, 463–467, 10.1603/0022-2585-40.4.463 (2003). [DOI] [PubMed] [Google Scholar]
- Dekker T., Ignell R., Ghebru M., Glinwood R. & Hopkins R. Identification of mosquito repellent odours from Ocimum forskolei. Parasites & Vectors 4, 10.1186/1756-3305-4-183 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller G. C. et al. Efficacy of the botanical repellents geraniol, linalool, and citronella against mosquitoes. Journal of Vector Ecology 34, 2–8, 10.1111/j.1948-7134.2009.00002.x (2009). [DOI] [PubMed] [Google Scholar]
- Sánchez-Bayo F. Insecticides mode of action in relation to their toxicity to non-target organisms. Journal of Environmental & Analytical Toxicology S4, 10.4172/2161-0525.S4-002 (2012). [DOI] [Google Scholar]
- Theiling K. M. & Croft B. A. Pesticide side-effects on arthropod natural enemies - a database summary. Agriculture Ecosystems & Environment 21, 191–218, 10.1016/0167-8809(88)90088-6 (1988). [DOI] [Google Scholar]
- Desneux N., Decourtye A. & Delpuech J. M. The sublethal effects of pesticides on beneficial arthropods. Annual Review of Entomology 52, 81–106, 10.1146/annurev.ento.52.110405.091440 (2007). [DOI] [PubMed] [Google Scholar]
- Mineau P. et al. Pesticide acute toxicity reference values for birds. Reviews of Environmental Contamination and Toxicology 170, 13–74 (2001). [PubMed] [Google Scholar]
- Raimondo S., Mineau P. & Barron M. G. Estimation of chemical toxicity to wildlife species using interspecies correlation models. Environmental Science & Technology 41, 5888–5894, 10.1021/es070359o (2007). [DOI] [PubMed] [Google Scholar]
- Fessl B., Kleindorfer S. & Tebbich S. An experimental study on the effects of an introduced parasite in Darwin’s finches. Biological Conservation 127, 55–61, 10.1016/j.biocon.2005.07.013 (2006). [DOI] [Google Scholar]
- Knutie S. A., McNew S. M., Bartlow A. W., Vargas D. A. & Clayton D. H. Darwin’s finches combat introduced nest parasites with fumigated cotton. Current Biology 24, R355–R356 (2014). [DOI] [PubMed] [Google Scholar]
- Sukumar K., Perich M. J. & Boobar L. R. Botanical derivatives in mosquito-control - a review. Journal of the American Mosquito Control Association 7, 210–237 (1991). [PubMed] [Google Scholar]
- Hardin J. A. & Jackson F. L. C. Applications of natural products in the control of mosquito-transmitted diseases. African Journal of Biotechnology 8, 7373–7378 (2009). [Google Scholar]
- Reader S. M. & Laland K. N. Animal innovation (Oxford university Press, 2003). [Google Scholar]
- Kummer H. & Goodall J. Conditions of innovative behavior in primates. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences 308, 203–214, 10.1098/rstb.1985.0020 (1985). [DOI] [Google Scholar]
- St Amant R. & Horton , T. E. Revisiting the definition of animal tool use. Animal Behaviour 75, 1199–1208, 10.1016/j.anbehav.2007.09.028 (2008). [DOI] [Google Scholar]
- Bentley-Condit V. K. & Smith E. O. Animal tool use: current definitions and an updated comprehensive catalog. Behaviour 147, 185–A132, (2010). [DOI] [Google Scholar]
- Shumaker R. W., Walkup K. R. & Beck B. B. Animal tool behavior: the use and manufacture of tools by animals. Revised and updated edition edn (Johns Hopkins University Press, 2011). [Google Scholar]
- Hunt G. R., Gray R. D. & Taylor A. H. Why is tool use rare in animals? Tool Use in Animals: Cognition and Ecology 89–118, Book_Doi10.1017/Cbo9780511894800 (2013). [Google Scholar]
- Gagliardo A. Forty years of olfactory navigation in birds. Journal of Experimental Biology 216, 2165–2171, 10.1242/jeb.070250 (2013). [DOI] [PubMed] [Google Scholar]
- Clark L. & Mason J. R. Olfactory discrimination of plant volatiles by the european starling. Animal Behaviour 35, 227–235, 10.1016/s0003-3472(87)80228-2 (1987). [DOI] [Google Scholar]
- Clark L. & Mason J. R. Sensitivity of brown-headed cowbirds to volatiles. Condor 91, 922–932, 10.2307/1368077 (1989). [DOI] [Google Scholar]
- Damiens D., Benedict M. Q., Wille M. & Gilles J. R. L. An Inexpensive and Effective Larval Diet for Anopheles arabiensis (Diptera: Culicidae): Eat Like a Horse, a Bird, or a Fish? Journal of Medical Entomology 49, 1001–1011, 10.1603/ME11289 (2012). [DOI] [PubMed] [Google Scholar]
- R: A language and environment for statistical computing. (R Foundation for Statistical Computing, Vienna, Austria, 2015).
- Hothorn T., Bretz F. & Westfall P. Inference in General Parametric Models. Biometrical Journal 50, 346–363 (2008). [DOI] [PubMed] [Google Scholar]
- Nojima S., Linn C., Morris B., Zhang A. J. & Roelofs W. Identification of host fruit volatiles from hawthorn (Crataegus spp.) attractive to hawthorn-origin Rhagoletis pomonella flies. Journal of Chemical Ecology 29, 321–336, 10.1023/a:1022677827233 (2003). [DOI] [PubMed] [Google Scholar]
- Cha D. H., Powell T. H. Q., Feder J. L. & Linn C. E. Identification of Host Fruit Volatiles from Three Mayhaw Species (Crataegus Series Aestivales) Attractive to Mayhaw-Origin Rhagoletis pomonella Flies in the Southern United States. Journal of Chemical Ecology 37, 961–973, 10.1007/s10886-011-0013-6 (2011). [DOI] [PubMed] [Google Scholar]
- Cha D. H., Adams T., Rogg H. & Landolt P. J. Identification and Field Evaluation of Fermentation Volatiles from Wine and Vinegar that Mediate Attraction of Spotted Wing Drosophila, Drosophila suzukii. Journal of Chemical Ecology 38, 1419–1431, 10.1007/s10886-012-0196-5 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.