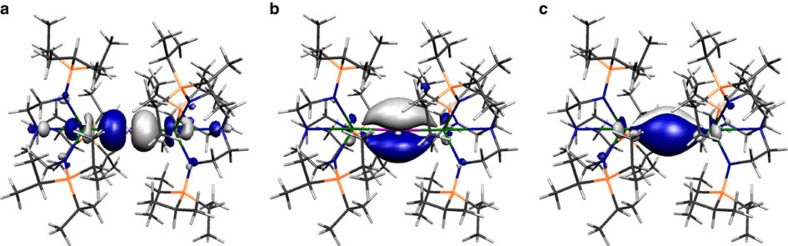
Figure 9. Kohn–Sham molecular orbital representations of the principal Th–P interactions of 6−.
HOMO−2 (a, −1.576 eV), HOMO−1 (b, −1.136 eV) and HOMO (c, −1.097 eV) represent the three principal thorium–phosphorus covalent σ- and π-bonding interactions in the anion component of 6. The two Th–P π-interactions are delocalized in the molecular orbital model but together with the σ-bond these pseudo triple bonds equate to Th=P double bonds in a Lewis bonding scheme.