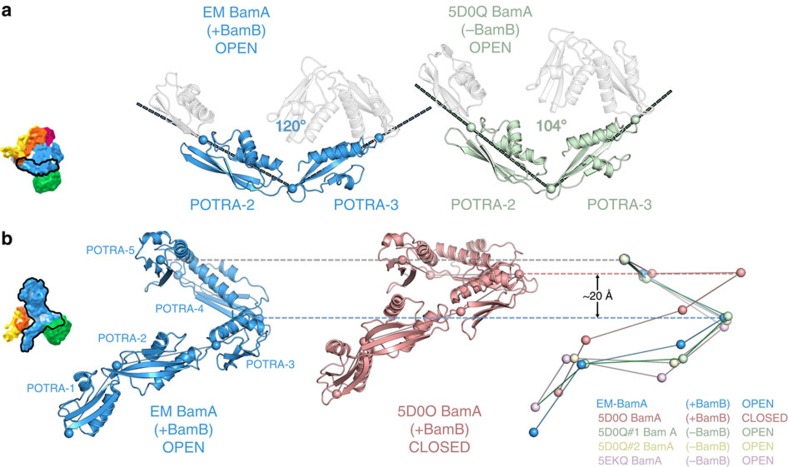
Figure 4. The effect of BamB binding and β-barrel conformation on the BamA POTRA domains.
(a) The presence of BamB correlates with a more obtuse angle between POTRA domains 2 and 3. On the left, is the EM structure (‘lateral open', +BamB; blue), and on the right 5D0Q33 (a ‘lateral open', -BamB, X-ray structure; pale green). The view is from outside the bacterial cell, looking approximately down the axis of the β-barrel (see thumbnail image). Both structures have the BamA β-barrel in a ‘lateral open' conformation; thus, the barrel opening and a wide POTRA 2–3 angle do not correlate, but BamB binding and a wide POTRA angle do correlate. The remaining POTRA domains are shown in pale grey. The angle is measured between identical points in the hinge regions between each POTRA domain, indicated by spheres. (b) Vertical extension of the POTRA domains correlates with β-barrel state. The open barrel of the EM structure (‘lateral open', +BamB; blue) and all ‘lateral open' X-ray structures, correlates with a vertically extended conformation of the POTRA chain, whereas the ‘lateral closed' barrel of 5D0O (+BamB; pink) has a much more compact POTRA chain. Both structures contain BamB; hence, BamB binding does not appear to correlate with the extension of the POTRA chain. Thumbnail images are in the appropriate view and coloured with the same colour scheme as Fig. 2. For overall comparisons of BamA, see Supplementary Fig. 9.