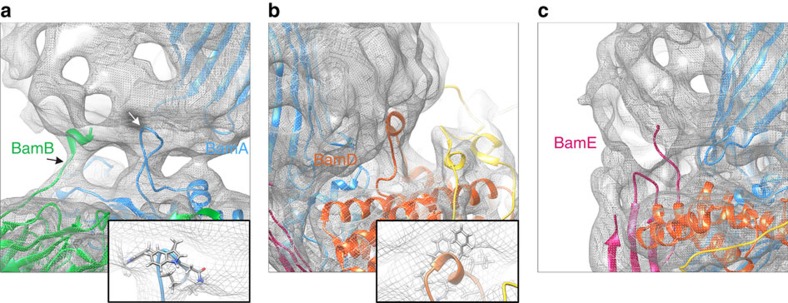
Figure 5. Interactions between BAM components and the detergent micelle.
(a) A hydrophobic loop within the body of BamA POTRA 3 (blue, white arrow) is buried within the micelle (grey mesh), with details of the hydrophobic residues inset. The N terminus of BamB (green, black arrow), which is unmodelled in the X-ray structures of BAM and its subcomplexes32,33,34, also dips into the micelle. (b) A hydrophobic 310 helix in BamD (orange) inserts into the micelle, with hydrophobic residues buried in the hydrocarbon tail groups of the detergent (see inset) and polar residues flanking the helix placed to interact with the polar head groups of the detergent. (c) The N terminus of BamE (magenta), which is the site of the lipid anchor, also inserts into the micelle, well away from the body of the BamA β-barrel.