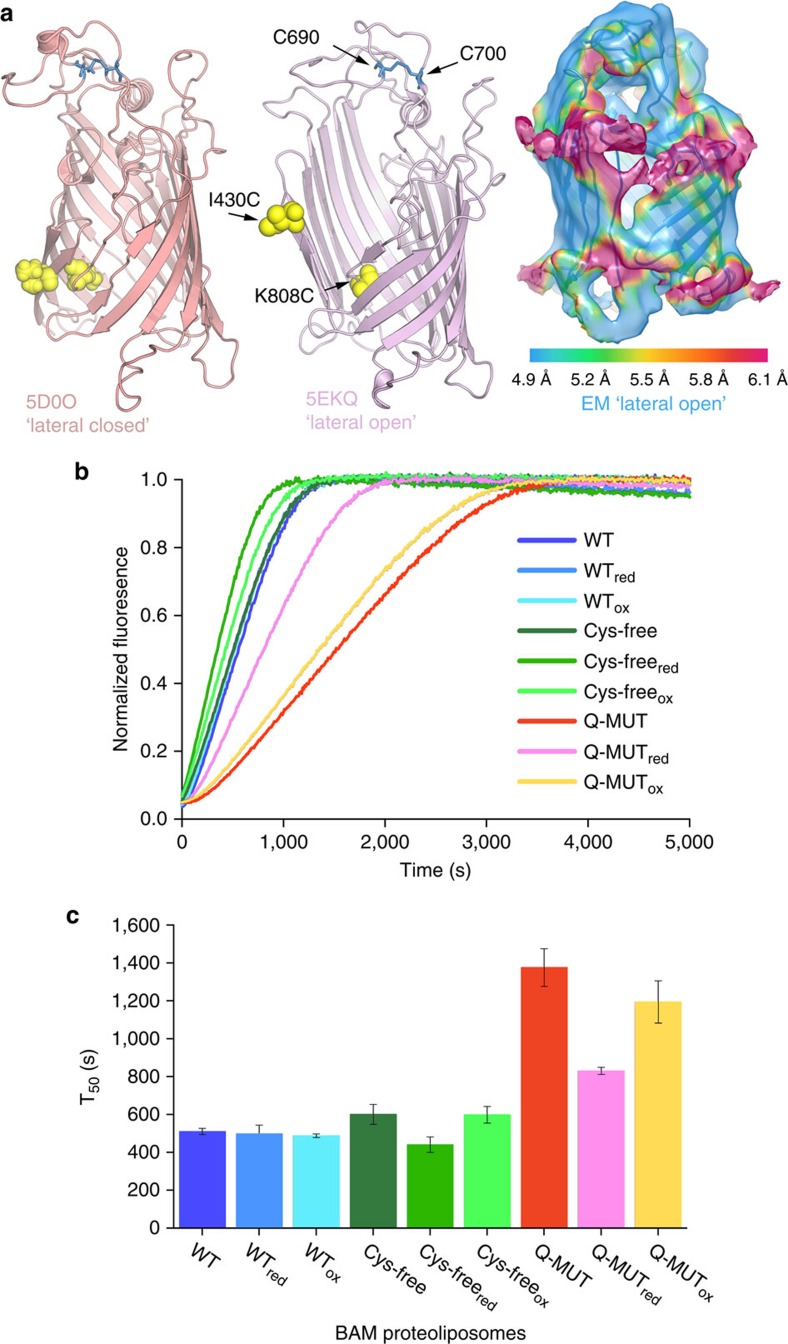
Figure 7. β-Barrel gating of BamA is required for full BAM activity in vitro.
(a) Cys residues introduced into the BamA β-barrel at positions 430 (I430C) and 808 (K808C) (yellow spheres) are hypothesized to be able to form a disulfide in the ‘lateral closed' structure (5D0O33) (left image), but not in a ‘lateral open' barrel (for example, 5EKQ32) (centre image). The two natural Cys residues (C690 and C700), which were removed, are shown in blue ball and stick. On the right hand side, the EM density is in a similar orientation and coloured according to local resolution63, showing that the density around β1–β16 is at a lower resolution and thus more mobile than the body of the barrel. (b) Example kinetic traces of OmpT folding measured by its proteolytic activity in the presence of BAM complexes containing wild-type BamA, BamAC690S/C700S (Cys-free) or BamAC690S/C700S/I430C/K808C (Q-MUT; see Supplementary Fig. 1). All experiments were performed with final concentrations of 0.25 μM BAM proteoliposomes, 5 μM OmpT, 1 mM fluorogenic peptide, 35 μM SurA, in oxidizing (1 mM CuSO4) or reducing (50 mM dithiothreitol (DTT)) conditions. All experiments were performed in 50 mM glycine-NaOH pH 9.5, 25 °C. (c) Bar chart of the average half-time for each folding reaction. These show the mean and s.e.m. from four repeats, across two proteoliposome preparations (see also Supplementary Table 3).