Abstract
Heart rate variability (HRV) corresponds to the adaptation of the heart to any stimulus. In fact, among the pathologies affecting HRV the most, there are the cardiovascular diseases and depressive disorders, which are associated with high medical cost in Western societies. Consequently, HRV is now widely used as an index of health.
In order to better understand how this adaptation takes place, it is necessary to examine which factors directly influence HRV, whether they have a physiological or environmental origin. The primary objective of this research is therefore to conduct a literature review in order to get a comprehensive overview of the subject.
The system of these factors affecting HRV can be divided into the following five categories: physiological and pathological factors, environmental factors, lifestyle factors, non-modifiable factors and effects. The direct interrelationships between these factors and HRV can be regrouped into an influence diagram. This diagram can therefore serve as a basis to improve daily clinical practice as well as help design even more precise research protocols.
Keywords: Cardiac coherence, Heart rate variability, Influence diagram, Literature overview
Introduction
“Life is about rhythm. We vibrate, our hearts are pumping blood, we are a rhythm machine, that’s what we are.” - Mickey Hart. With its strategic position, the heart acts not only as the main pump but also as a global coordinator in synchronism with the other body functions. Because of the influence of many factors, the heart does not maintain the exact same rhythm from one heartbeat to the next. It reacts to any stimulus by increasing or decreasing its rhythm so that the body can adapt to any change.
This adaptation is called heart rate variability (HRV) and can also be characterized in terms of amplitude: weak, normal or high. A high HRV is the sign that the heart is healthy since it has more flexibility to react to any stress (physiological or environmental). Alternatively, a reduced HRV is a symptom of health problems and can affect immune functions, self-regulation and psychosocial abilities (1). Among the pathologies affecting HRV the most, there are the cardiovascular diseases (CVD) and depressive disorders.
One can therefore wonder what kind of factors can directly influence HRV. Actually, many specific reviews already focused on HRV in order to described in precise details how specific brain areas are affecting HRV, how heart dysfunctions can influence individual health, how respiration induces heart acceleration or deceleration, how stress factors act on the heart, and so on. There were very numerous studies, such that HRV is now commonly used as a health indicator.
Despite all of these numerous studies and reviews, none was carried out to regroup all the information. The originality of this literature review is to analyze the numerous previous reviews and meta-analyses in order to present an overview of the problem, to create a comprehensive map of the main factors influencing HRV. Knowing all the environmental and physiological factors and how they can influence amongst themselves will help to build more precise research protocols and also help clinicians in their daily practice for treatment and recommendations to their patients. Actually, with the specific methodology described below, the categorization phases of this study could point out five main themes among the counted elements: physiological and pathological factors, lifestyle factors, non-modifiable factors, neuropsychological factors and environmental factors. These categories will be detailed and a synthesis of the links between them will be further presented with a diagram of influences.
Methods
This literature review was carried out according to a systematic protocol, commonly used (2, 3). With search tools such as PubMed, Medline, and Scopus, the literature search was conducted in summer 2015 with the following keywords in combination: heart rate, coherence, variability, physiology, heart brain connection, electromagnetic field, blood pressure, cerebral, emotions, neurocardiology. Several scientific validity criteria were verified for each original article: presence of controls, randomization, statistical analysis, and results’ significance.
Systematic review articles as well as meta-analyses were first consulted. References in these articles were then consulted in order to identify additional studies. Following this step, in order to refine the results, a descending method was used from recent secondary sources. In front of the very high number of studies, it was then needed return to an ascending method allowing the results’ refinement and to maintain the update.
The exclusion of articles was mainly based on the relevance to the subject regarding to the research question, thus reducing the interpretation biases and deficient protocols. The second selection criterion followed the proof level pyramid according to the scientific validity of each study, mentioned above. Over 150 reviews and articles were analyzed, 87 were finally selected and 56 are presented within this article. Any article that could not explicitly reveal the needed scientific validity criteria, such as abstracts or conference papers, was excluded.
Influence diagram
An influence diagram aims to graphically represent the statistically significant cause-to-effect links between the different elements as well as with the harmful effects. There are several techniques, based on causes, consequences or events, allowing linking of the causes to the consequences. The method chosen for this literature overview was based on the root cause analysis tree creation in order to identify the direct actors influencing HRV (4). This approach is simple to use, favors the systematic aspect of research of causes and their links, and does not need chronological relationships between the factors and their effects.
The creation of the influence diagram was carried out using an iterative process in 4 steps (Fig. 1).
Fig. 1 -.
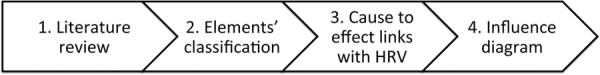
Schematization of the method used for the creation of a diagram of influence towards heart-rate variability (HRV).
-
-
Step 1: Literature review and analysis.
-
-
Step 2: Categorization. Two levels of classification were used:
-
•
First, all elements could be undertaken whether the factor was associated with an intrinsic parameter (source), a transfer towards the target (exposure) or a harmful effect (target). In the influence diagram, a cause is connected to an effect by a single-direction arrow. Some elements could not be assimilated to a cause or an effect since they could be both. In this case, double direction arrows are to be used.
-
•
Second, all the identified elements were regrouped into five different specific categories, detailed further in the Results section.
-
•
-
-
Step 3: Establish the nature of cause to effect links (beneficial, harmful or undefined) between the identified elements and HRV. Different types of arrows were used in the diagram to account for this aspect. Details are given in the figure legend.
-
-
Step 4: All data and observations during this step were then compiled and structured for all elements, as well as for the links uniting them, in the form of an influence diagram (step 4), by using the details described in the previous steps.
Results
Five main categories could be identified through the literature analysis: physiological and pathological, neuropsychological, lifestyle, non-modifiable and environmental factors. Beneficial and deleterious effects of high and low HRV were presented, respectively, when possible. Each category was detailed accordingly with significant conclusions in order to determine the cause-to-effect relationship between each factor and HRV and if these links could be considered as significantly harmful or beneficial, or as not significant enough to draw a conclusive remark. In such cases, recommendations were made, respectively.
Heart rate variability and neurological pathways
Cardiac innervation
Briefly, the heart intrinsic neural network is composed of many subpopulations of specialized cells (nodes) able to generate electric currents spontaneously while the extrinsic of cardiac innervation regroup the efferences and afferences of the autonomic nervous system (ANS) with the sympathetic (SANS) and parasympathetic (PANS) (mainly the vagus nerve [X]) divisions (5).
Central control of HRV
Besides X’s nuclei, several endocranial structures play a preponderant part in HRV modulation. In different literature reviews and MRI studies, Thayer et al (6-7-8-9), could identify the main ANS actors linked with HRV.
Moreover, Thayer et al (6, 7, 10), through reviews and meta-analyses over 10 neuroimaging datasets, confirmed the regions consistently associated with HRV and even proposed a neurovisceral integration model to better understand the neuronal activation and inhibition effects of ANS actors on the heart. On another side, the recent review proposed by McCraty and Childre (11) also resulted in the presentation of the known afferences from the heart and vascular system towards the CNS.
By combining the heart anatomy (5) with the structures identified by Thayer et al and by McCraty et al, Figure 2 was built to briefly schematize the main actors involved in HRV modulation, including the hypothalamus, nucleus tractus solitarii (NTS, nucleus of the solitary tract), periaqueductal gray matter, amygdala, thalamus, dorsal vagal complex, prefrontal, insular and cingulate anterior cortices.
Fig. 2 -.
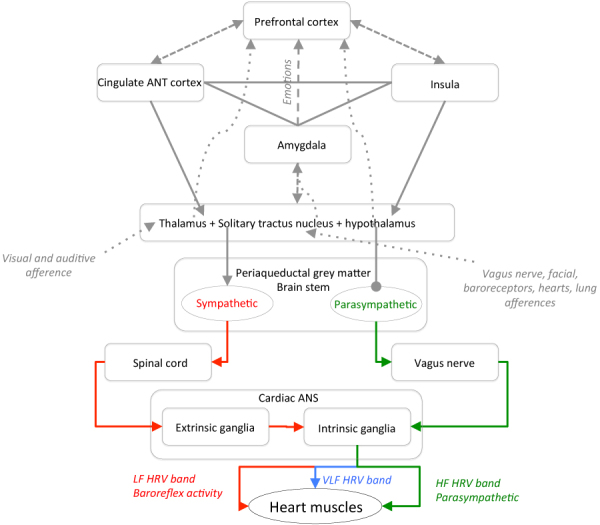
Schematization of the afferences and efferences between some ANS actors and HRV. Round dotted arrows indicate afferences, plain arrows indicate efferences while squared dotted arrows indicate both ways communication between the related structures. Low frequency (LF) band (red arrows) corresponds to the sympathetic share of the HVR specter, while the high frequency (HF) band (green arrows) is associated with the parasympathetic share, distributed through the vagus nerve mainly. Very low frequency (VLF) band (blue arrows) is still under consideration but can be related to the cardiac autonomous nervous system (ANS).
Such schematic representation already suggests the complexity of heart-brain connections but can help clinicians to identify neurological disorders resulting in HRV decreases. Moreover, even if many structures were identified, the underlying mechanisms linking the heart to the brain (and reciprocally) are more complex and yet to be fully elucidated (9).
Heart rate variability analysis
Temporal analysis
The other and the simplest way to analyze HRV data is in the carry out time domain method. Even if it does not provide any quantifiable insight in the autonomous tone and balance, it is still widely used and recognized among the scientific community, thus allowing comparisons of datasets. In fact, different analytic parameters can be extracted, such as the standard deviation normal to normal or root mean square of successive differences. The latter was actually recognized to accurately estimate the changes in HRV due to nervous information carried out by the vagal nerve (12).
Frequency analysis
The representation of HRV basically comes from the report of RR intervals from electrocardiograms (ECGs) versus time. Many studies (13, 14) showed that HRV can be analyzed under two major frequency bands: high frequencies (HF) (0.15-0.4 Hz) and low frequencies (LF) (0.04-0.15 Hz), associated with ANS. While LF was found to be a poor marker of sympathetic tone but was rather related to baroreflex activity (15), HF seems to consistently correspond to parasympathetic tone (12, 16, 17). HRV can be used as a health index, a biofeedback tool and a mean to report on the ANS-CNS integration (8).
Over recent years, and with Dr J. Andrew Armour research, a new theory suggests that the network regrouping all the cardiac neurons, neuronal connections, and the neurotransmitters, actually constitutes a brain as such. This complex circuit is able to manipulate information independently of central and autonomous controls, and was correlated with the very low frequency part of the spectrum (0.0033-0.04 Hz) (18-19-20-21).
Another band, the ultra-low frequency band (below 0.0033 Hz), can only be extrapolated with 24-hour recordings (22). Yet their physiological components need to be fully determined. Consequently, based on this limited information, future investigations are needed, while the major bands might be more useful tools for daily clinical assessment of HRV.
Cardiac coherence
While physiological HRV is represented by an irregular frequency versus time curve (named as chaotic), a specific state (often due to a regular or controlled respiration) induced the heart rate to describe a sinusoidal curve around 0.1 Hz. This state is referred as coherence or resonance. The spectral analysis of such state gives a high peak in the LF band and none in the HF or VLF bands. According to McCraty and colleagues at the Heart Math Institute (HMI), heart coherence is a manifestation of physiological coherence (a term introduced by these researchers) and allows the improvement of individual self-regulatory processes (12).
The primary objective of this review is to list the factors directly linked to HRV. The analysis of the effects of cardiac coherence (CC), in addition to increase physiological amplitude of HRV and reduce stress, was already carried out in detail by the HMI researchers. One of their reviews on the subject was published in 2015 and describes with precision the benefits of CC (23). Therefore, when applicable, only specific effects of CC are mentioned accordingly throughout this article.
Physiological and pathological factors
Recently, it was analyzed, reviewed and concluded that a low HRV, through deficient cholinergic reflexes or through pro-inflammatory cytokines, in the long term leads to immune dysfunction and inflammation in addition to CVD or depression (1, 8). Besides the nervous actors and these reflexes, other physiological elements and their pathological correspondents can influence the HRV: endocrine, respiratory, cardiovascular and neurological factors.
Endocrine factors
By considering the reciprocal messages between the heart and the rest of the body, the hormones are amongst the messengers worth mentioning and briefly detailed.
Although their potential benefits to patients affected by CVDs (24), a recent controlled study reported that thyroxin treatments for epithelial cancers can decrease HRV significantly (25), actually confirming recent results on hypothyroidism (26). In fact, thyroid hormones have direct effects on the myocardium by increasing its contractility but also on the ANS by altering the sympathetic response (27).
On another side, estrogen levels were significantly correlated with HRV measures in healthy women, thus confirming the cardioprotective effect of feminine sexual hormones (28). Additionally, masculine androgens have a beneficial effect (parasympathetic) on the heart autonomous modulation (higher HRV with high testosterone levels) (29) while estradiol tends to preferably induce a parasympathetic activity (30). Consequently, even if a literature analysis confirmed that women tend to have a greater parasympathetic response capacity than men, who seem to be more sympathetically responsive (31), the mechanisms underlying the relationship between HRV and sexual hormones are yet to be completely elucidated.
Moreover, a preliminary study (32) reported a decrease in salivary cortisol for subjects practicing self-emotional management program, correlated with in an increase in coherent state in HRV signals.
Based on the analyzed studies, it would seem that the relationships of the thyroid, sexual hormones or cortisol with HRV are established and significant in some cases, therefore useful for such investigation, but their nature seems rather complex and needs to be clarified with future extended and specific review.
Respiratory factors
Another factor greatly influencing HRV is due to thoracic respiration (33). A physiological phenomenon happens at each respiration: the respiratory sinus arrhythmia (RSA) where heartbeat increases as the air is breathed in and decreases at each expiration.
In 1999, van Ravenswaaij-Arts et al (33) proposed a simple schematization of the heart-brain interactions, involving many actors participating in RSA, such as baroreflexes, blood pressure (BP) and chemoreceptors. More recently, a literature review detailed two main mechanisms to describe RSA (34), involving tonic and phasic chemo- and, barometric reflexes, heart and lung stretching reflexes, some local metabolic factors, central generators of heart rhythm and the corresponding nerves (X particularly) (35). Such studies underlined different structures involved in heart-brain interactions and influencing HRV. It is however important to mention that RSA is also influenced by several factors (age, gender, ethnicity, posture, cardio-pulmonary function), these same variables can also in turn affect HRV (34).
Based on these models (33, 34), it is logical to stipulate that respiratory pathologies may in turn affect HRV. In fact, and for example, asthma has been linked to reduced HRV in children (36).
Neurological factors
As presented previously, neural connections between the heart and the brain are numerous. If one of these structures is affected, it is logical to expect that HRV will be as well. In fact, neurological disorders, associated with brain damage, were linked with an HRV decrease: Parkinsonian syndrome, spinocerebellar degeneration, Shy-Drager syndrome, multiple sclerosis, Guillain-Barré syndrome (33).
In turn, not only cardiac coherence (CC) biofeedback could equilibrate ANS, favor immune function, but also could improve academic performances and other cognitive functions such as memory (11, 23). A recent transversal study suggested that CC could possibly facilitate neurocortical reorganization, even if would be difficult to confirm (37).
Consequently, based on all these results, one could possibly hypothesize that increasing HRV could potentially have a beneficial effect on the symptoms, on the rate of the neurological disease, or at least on the risk factors to develop such disorders.
Cardiovascular diseases (CVD)
To be able to detect CVD is as important as understanding their etiology. For many years, several research teams have used HRV as an indicator of these types of pathologies.
In their systematic review, Thayer et al (38) studied in detail the risk factors of CVD and noted that epidemiological studies tend to confirm that HRV is lower for subjects with high BP rather than for subjects with normal BP. A similar relationship could be established with a high level of blood cholesterol or glucose (diabetes) and a decreased HRV.
This review coupled with other studies and reviews confirmed that a decreased HRV is an indicator of CVD (1, 14, 38-39-40). Therefore, it was not relevant to detail further the now recognized relationship between CVD and low HRV.
Other factors
The model proposed by van Ravenswaaij-Arts et al (33) involves the renin-angiotensin system and also the thermoregulation. A literature review of HRV identified many parameters influencing HRV, such as body temperature. In fact, if the temperature decreases enough, it induces a bradycardia (this phenomenon is actually applied for open-heart surgery) (41). Alternatively, when temperatures rise, such as with fever, heart beats accelerate. Consequently, this aspect points out that the ANS and CNS are not the only regulators of HRV - other physiological factors are also involved.
Lifestyle factors
Physical activity
Interestingly, significant results, analyzed by several literature reviews, were obtained to validate the increase of heartbeat from lying down position to seated position and from seated to standing up positions (14, 33, 39).
Physical activity, which action can vary from one individual to another, is known to change HRV (39) by decreasing sympathetic activation (40). The Whitehall study (42) noted that HRV was found to be higher (and cardiac rhythm lower) in subjects having physical activity from moderate-to-vigorous.
Moreover, a quantitative link was established between the ultra-low frequency band and muscle activity (43). Voluntary restriction of physical activity resulted in a decrease of this band power, thus suggesting the need to include physical activity in future ambulatory HRV studies. As such, a recent review suggested that supervised and non-supervised exercise therapy could increase HRV in patients affected by CVDs and diabetes mellitus (44). The authors pointed out the possible implication of angiotensin and Nitric Oxide (NO) in the physiological mechanisms as well as the need for further understanding of such mechanisms.
On the opposite side, even if the short-term and long-term effects of training in athletes remain to be clarified, this population can show various HRV profiles, thus confirming the usefulness of HRV as a tool to monitor their physiology. However, much care in methodology is needed when designing analyzing studies with athletes (45).
Consequently, based on these observations, it is safe to assume, given the other known benefits of exercise therapy, that this factor can improve HRV in populations varying from CVD patients to athletes.
Alcohol, tobacco and drug consumption
Alcohol and tobacco consumption also have an effect on HRV. Chronic smokers and alcohol drinkers have a low HRV but this effect is reversible when they stop drinking alcohol or smoking (38, 39).
Regarding alcohol, it induces an HRV decrease, which could be related to a sympathetic activation or a parasympathetic inhibition (14). This observation was confirmed in over 542 healthy subjects separated into two groups according to their alcohol consumption level (high or low) (46). In fact, this study revealed that high alcohol consumption is associated not only with a decreased HRV, but also with a high cortisol level, suggesting the possible implication of the hypothalamo-hypophyseal-adrenal axis in this phenomenon.
There are several studies on the use of different medication and drugs and some examples can show how drugs can influence HRV. In particular, a quantitative review on antidepressants revealed that tricyclic medication significantly decreased HRV in studies on which recording was carried out on short time intervals (47). Depending on medication type and population, results can greatly vary. Besides, a quantitative review concluded that the necessary sample to obtain significant HRV changes could need 660 patients taking conventional medication to treat coronary dysfunctions (beta-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors) (48). Necessarily, the cause-to-effect link between medication and HRV was established but its nature would need to be clarified with large-scale populations and with different category of medication. Since the reverse beneficial effect of CC on tobacco users was studied, it would also be interesting to replicate the experiments with heavily medicated subjects.
Meditation
Recent studies reported that HRV could be used as an indicator of meditative state (37). Moreover, in a transversal study in over 14 subjects in 2014, they reported that autogenic meditation (also called “Schultz’s autogenic training”) leads to an increase of CC score (ratio of medium frequencies over the sum of LF and HF), and of alpha, beta and gamma brainwaves. According to these results, it indicates a stronger structural or functional connection between cortical and thalamic regions, as previously presented (Fig. 2). In a transversal study (49), the coherence ratio (HF/LF) significantly increased after a meditation practice by Vipassana experts. This increase was also correlated with a greater attention commitment and a reinforced autonomous regulation (ANS).
Non-modifiable factors
With age, HRV decreases. This is a known fact confirmed by a quite recent systematic literature review (38). Even if the underlying mechanism is yet to be elucidated, some researchers think that it might be related to ANS maturation and that this decrease seems to affect women more than men (14).
No specific study was specifically dedicated to the influence of ethnicity on HRV. However, the systematic review of Thayer et al (38) mentioned that the studies they analyzed were normalized with age, gender and ethnicity. Indirectly, this indicates that ethnicity (or more generally genetics) has an influence on BP and consequently on HRV. This aspect is now well known by the scientific community without need to be a detailed object of this literature overview.
To the authors’ knowledge, the literature observing the effect of genetics on HRV is relatively limited. Nevertheless, two studies were conducted to examine the heritability of HRV and the results highlighted the contribution of genetics within the ANS activity (50, 51). Another study pointed out that ethnic differences have an effect on HRV during youth, while no differences were observed for gender (52). Consequently, whilst heritability seems to be a factor to be accounted for when analyzing very specific traits on HRV, this relationship needs further clarification and future research.
Environmental factors
A recent systematic review actually established a detailed list of work-related factors that can influence HRV (53). Among these factors there is particulate matter, several chemical components, electromagnetic fields (EMF), vibrating tools, psychosocial charge, working time, fatigue, etc. A prolonged exposure to these factors leads to a decrease in HRV.
Further literature analyses would need to examine the effect of these factors on the specific physiological structures identified by Thayer and coworkers and others, but also on the other categories presented here, in order to understand the underlying mechanisms of such effect.
Considering that modern lifestyles involve the use of many electronic devices and wireless connections and that HMI researchers pointed out the interaction between electromagnetic human heart fields (11), the daily exposure to EMF generated by electronic devices and their effect on human physiology should also be the subject of future research.
Neuropsychological factors
Another group of endocranial structures was identified and behavior (social, intentional, affective, executive) by modulating motivation from external and internal stimuli (54). Among this network, there are the anterior cortex, the amygdala, the insula, the periaqueductal grey matter, which are also structures regulating HRV (as pointed out by Fig. 2). Consequently, and from a general point of view, HRV is linked to personality but a list of neuropsychological factors influencing HRV is not limited to this aspect and actually includes stress, depression and negative emotions.
Stress
With a brief research on stress, one can realize quickly that numbers contradict each other, without further details. However, stress can be the most dominant factor, and it needs to be mentioned that the evaluation of stress should be standardized, which is still not the case, since stress is multifactorial (10).
With a systematic approach, it was possible to list many stress sources inducing low HRV, such as anxiety, hostility, depression, but an emerging source recently gained attention - work stress. In fact, many analyzed studies revealed a significant correlation between a high level of work stress and low HRV. This remains the case even by using several variables (gender, alcohol consumption, education level, work characteristics, etc.). Besides, the same stress was also correlated with a higher risk of CVD and a high cardiac rhythm (38).
Depression
From a recent meta-analysis, Kemp et al (55) obtained a significant correlation (p<0.001) between depression and a low HRV. They also verified the effects of many antidepressants and the result was identical for tricyclic medication. They also emphasized for the need for long-term studies.
The low HRV value, even if depression is not associated with a CVD, remains an indicator of poor health (23, 55). However, the affect is reversible in this case. In fact, in a controlled transversal study on 24 subjects, a significant (p<0.05) return to a higher HRV was observed when depressed patients used a biofeedback approach.
Negative emotions
In psychology, neuroticism is associated with the experience of negative emotions and concerns patients suffering from anger, depression or anxiety (56). A transversal study on 33 healthy subjects confirmed the significant correspondence between weak neuroticism (negative emotions) and low HRV and the inverse as well (57).
While negative emotions, such as anger, anxiety, frustration and worry produce every irregular ECG and a reduced HRV, it is also worth mentioning the benefits obtained by negative emotions by practicing CC (23). Even if the underlying mechanism remains unknown, the reversibility of cardiovascular after-effects (due to negative emotions) by positive emotions was first demonstrated in 2000 and then confirmed in 2010 by Fredrickson and Levenson (58). Kemp and Quintana (1) also concluded that HRV holds a significant functional importance on social behavior, self-regulation and psychological flexibility against life stressors.
Discussion
Themes identified
Synthesis of literature review
The data analyzed in this exhaustive literature review on physiological, environmental and psychological elements linked with HRV are regrouped by category and presented in Figure 3, with an influence diagram. The aim of this diagram is to synthesize the information schematically in order for it to be used for research protocols, eventually.
Fig. 3 -.
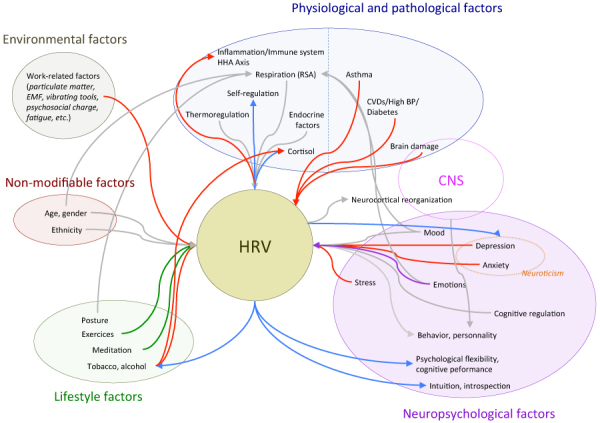
Influence diagram of cause of effect factors linked with heart-rate variability (HRV). The direction of the arrows indicates a cause-to-effect link between related factors. While red arrows indicate a deleterious effect and green or blue arrows a beneficial one, purple arrows refer to a link for which the effect can be deleterious or beneficial. Blue arrows are specific to the heart coherence state. Red, green and blue arrows correspond to significant effects while grey arrows indicate a link for which statistical significance was not achieved.
This diagram emphasizes the importance of the brain-heart interaction from the additional connections and the overlapping between physiological and neuropsychological factors. In fact, besides the multiple nervous links (Fig. 2), heart and brain also communicate through neurotransmitters, hormones but also through EMF. As observed and confirmed by (23), electromagnetic waves generated by the heart in coherent state are immediately recorded by brain waves. The subject of EMF is still poorly studied and needs specific and expensive instruments.
This heart-brain communication could also be involved in neurodegenerative diseases (NDD). In fact, even if the mechanisms remain uncertain, the analyzed research proves that there is a vascular etiology between Alzheimer’s disease (AD) and vascular and cardiac dysfunctions (59). The same author even questions the classification of AD under the NDD category and suggests that AD be seen as a CVD (60). According to their literature review, one of the potential mechanisms would involve the incorrect folding of proteins. The authors even suggest the term “Alzheimer’s cardiac disease” to name some CVD. These observations bring us to think if brain degeneration is a cause or a consequence of NDD.
Complexity of the system and originality of the research
As presented by Figure 3, HRV surrounding system is multifactorial. From the selected literature, some links already appear, such as between respiration and emotions or posture, or mood or age. The fact is, respiration holds a particular key role in the regulation of HRV. Since it is a physiological parameter that anyone can control, it seems therefore natural that such parameter is the base of many meditative techniques designed to improve HRV by favoring coherent states.
Besides, since literature underlines, many interactions exist between HRV and the central nervous system (CNS). Moreover, some neuropsychological factors can involve other CNS elements, which can influence the identified elements of ANS. Experts in this domain, already acknowledge that not all the mechanisms are well described and understood and that much research is needed in order to clarify the implication of CNS and its links with the heart.
Most selected texts are reviews and meta-analyses relatively specialized but very detailed in each domain. The originality of this research is found in the synthesis and compilation of all the scattered information and its potential usefulness for daily clinical practice as well as for designing methodologically precise research protocols.
Other aspects reflecting the existing literature
In fact, most of research conducted on HRV consists of crossed or transversal studies, valid but easier to carry out. In both cases, statistical tests are necessary to obtain trustful results. In transversal studies, the tested populations are only a sample of the general population at a given time, leading to difficulties in generalizing and extending the results. For crossed studies, the lack of a control group brings issues for results comparison. This aspect underlines, as many researchers suggested (53), a lack of longitudinal studies, in order to know the long-term effects (harmful or beneficial) of these factors on HRV.
Critic on research
As mentioned earlier, the originality of this study can be found in the gathering of scattered data from each respective domain and to present the main factors directly influencing HRV into a visual and easily usable representation. In front of the magnitude of the work, it was not possible to exhaustively determine the indirect links between the different categories. Consequently, future research in this area should focus on identifying the reinforcement links and to further detail the influences between each category. Even if some factors need further investigation, the identified link could still be of use for daily clinical assessment.
The limit accorded to this study in terms of the identification of the direct links only, cannot consequently show the strengthening effects due to the multiple possibilities of relationships between each different factor. However, this limitation also could eliminate any overflow towards ANS since HRV could be used as an indicator of ANS balance. Moreover, the mechanisms linking HRV and others factors are still poorly understood and much is to understand if the HRV changes are related to these different factors in a causal or effective manner.
Internal validity can be obtained with a more detailed analysis of several studies in order to confirm the links established in Figure 3. External validity cannot be obtained since too many inter- and intra-category links remain uncertain. A meta-analysis could be adequate to confirm the reliability of such diagram in front the unique and multifactorial complexity of such a system.
Conclusion and future perspectives
As summarized by this study, the group of factors influencing HRV is multifactorial and could give a preliminarily exhaustive portrait of this system thanks to the influence of diagram creation The main categories standing out from this study (physiological/pathological, neuropsychological, non-modifiable, lifestyle and environmental factors) are interdependent, hence the complexity of the system. Such interdependency definitely needs to be further detailed.
Disclosures
Financial support: The authors would like to thank the Fondation canadienne pour l’enseignement et la recherche en ostéopathie for participating in the publishing fees.
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Kemp AH, Quintana DS. The relationship between mental and physical health: insights from the study of heart rate variability. Int J Psychophysiol. 2013;89(3):288–296. doi: 10.1016/j.ijpsycho.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 2.Randolph JJ. A Guide to writing the dissertation literature review. Practical assessment, research and evaluation. 2009;14(13):1–13. [Google Scholar]
- 3.Boote DN, Beile P. Scholars before researchers: on the centrality of the dissertation literature review in research preparation. Educational Researcher. 2005;34(6):3–15. [Google Scholar]
- 4.Wilson PF, Dell LD, Anderson GF. Root cause analysis: a tool for total quality management. Milwaukee, Wisconsin: ASQ Quality Press; 1993 [Google Scholar]
- 5.Laske TG, Shrivastav M, Iaizzo PA. The cardiac conduction system. In: Iaizzo PA, ed. Handbook of cardiac anatomy, physiology and devices. Springer Science+Business Media. LLC. 2009;11:159–175. [Google Scholar]
- 6.Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev. 2009;33(2):81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61(3):201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- 8.Thayer JF, Brosschot JF. Psychosomatics and psychopathology: looking up and down from the brain. Psychoneuroendocrinology. 2005;30(10):1050–1058. doi: 10.1016/j.psyneuen.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Lane RD, McRae K, Reiman EM, Chen K, Ahern GL, Thayer JF. Neural correlates of heart rate variability during emotion. Neuroimage. 2009;44(1):213–222. doi: 10.1016/j.neuroimage.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 10.Thayer JF, Åhs F, Fredrikson M, Sollers JJ, III, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. 2012;36(2):747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 11.McCraty R, Childre D. Coherence: bridging personal, social, and global health. Altern Ther Health Med. 2010;16(4):10–24. [PubMed] [Google Scholar]
- 12.McCraty R, Shaffer F. Heart Rate Variability: New Perspectives on Physiological Mechanisms, Assessment of Self-regulatory Capacity, and Health risk. Glob Adv Health Med. 2015;4(1):46–61. doi: 10.7453/gahmj.2014.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad S, Tejuja A, Newman KD, Zarychanski R, Seely AJ. Clinical review: a review and analysis of heart rate variability and the diagnosis and prognosis of infection. Crit Care. 2009;13(6):232–238. doi: 10.1186/cc8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajendra Acharya U, Paul Joseph K, Kannathal N, Lim CM, Suri JS. Heart rate variability: a review. Med Biol Eng Comput. 2006;44(12):1031–1051. doi: 10.1007/s11517-006-0119-0. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein DS, Bentho O, Park M-Y, Sharabi Y. Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Exp Physiol. 2011;96(12):1255–1261. doi: 10.1113/expphysiol.2010.056259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Napadow V, Dhond R, Conti G, Makris N, Brown EN, Barbieri R. Brain correlates of autonomic modulation: combining heart rate variability with fMRI. Neuroimage. 2008;42(1):169–177. doi: 10.1016/j.neuroimage.2008.04.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Åhs F, Sollers JJ, III, Furmark T, Fredrikson M, Thayer JF. High-frequency heart rate variability and cortico-striatal activity in men and women with social phobia. Neuroimage. 2009;47(3):815–820. doi: 10.1016/j.neuroimage.2009.05.091. [DOI] [PubMed] [Google Scholar]
- 18.Rosen MR, Cohen IS, Danilo P, Jr, Steinberg SF. The heart remembers. Cardiovasc Res. 1998;40(3):469–482. doi: 10.1016/s0008-6363(98)00208-9. [DOI] [PubMed] [Google Scholar]
- 19.Huang M-H, Friend DS, Sunday ME et al. An intrinsic adrenergic system in mammalian heart. J Clin Invest. 1996;98(6):1298–1303. doi: 10.1172/JCI118916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armour JA. Neurocardiology - Anatomical and functional principles, 2003, HeartMath Research Center, Institute of HeartMath: Boulder Creek, CA. Publication No. 03-011 [Google Scholar]
- 21.Armour JA, Kember GC. Cardiac sensory neurons. In: Armour JA, Ardell JL, eds. Basic and clinical neurocardiology. New York: Oxford University Press. 2003:79–117. [Google Scholar]
- 22.Kleiger RE, Stein PK, Bigger JT., Jr. Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol. 2005;10(1):88–101. doi: 10.1111/j.1542-474X.2005.10101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCraty R, Atkinson M, Tomasino D, Bradley RT. The coherent heart heart–brain interactions, psychophysiological coherence, and the emergence of system-wide order. Integral Review. 2009;5(2):10–115. [Google Scholar]
- 24.Fazio S, Palmieri EA, Lombardi G, Biondi B. Effects of thyroid hormone on the cardiovascular system. Recent Prog Horm Res. 2004;59(1):31–50. doi: 10.1210/rp.59.1.31. [DOI] [PubMed] [Google Scholar]
- 25.Shuvy M, Arbelle JE, Grosbard A, Katz A. A simple test of one minute heart rate variability during deep breathing for evaluation of sympatovagal imbalance in hyperthyroidism. Isr Med Assoc J. 2008;10(8-9):603–606. [PubMed] [Google Scholar]
- 26.Celik A, Aytan P, Dursun H et al. Heart rate variability and heart rate turbulence in hypothyroidism before and after treatment. Ann Noninvasive Electrocardiol. 2011;16(4):344–350. doi: 10.1111/j.1542-474X.2011.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polikar R, Burger AG, Scherrer U, Nicod P. The thyroid and the heart. Circulation. 1993;87(5):1435–1441. doi: 10.1161/01.cir.87.5.1435. [DOI] [PubMed] [Google Scholar]
- 28.Leicht AS, Hirning DA, Allen GD. Heart rate variability and endogenous sex hormones during the menstrual cycle in young women. Exp Physiol. 2003;88(3):441–446. doi: 10.1113/eph8802535. [DOI] [PubMed] [Google Scholar]
- 29.Wranicz JK, Rosiak M, Cygankiewicz I, Kula P, Kula K, Zareba W. Sex steroids and heart rate variability in patients after myocardial infarction. Ann Noninvasive Electrocardiol. 2004;9(2):156–161. doi: 10.1111/j.1542-474X.2004.92539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doğru MT, Başar MM, Yuvanç E, Simşek V, Sahin O. The relationship between serum sex steroid levels and heart rate variability parameters in males and the effect of age. Turk Kardiyol Dern Ars. 2010;38(7):459–465. [PubMed] [Google Scholar]
- 31.Dart AM, Du X-J, Kingwell BA. Gender, sex hormones and autonomic nervous control of the cardiovascular system. Cardiovasc Res. 2002;53(3):678–687. doi: 10.1016/s0008-6363(01)00508-9. [DOI] [PubMed] [Google Scholar]
- 32.McCraty R, Barrios-Choplin B, Rozman D, Atkinson M, Watkins AD. The impact of a new emotional self-management program on stress, emotions, heart rate variability, DHEA and cortisol. Integr Physiol Behav Sci. 1998;33(2):151–170. doi: 10.1007/BF02688660. [DOI] [PubMed] [Google Scholar]
- 33.van Ravenswaaij-Arts CM, Kollée LA, Hopman JC, Stoelinga GB, van Geijn HP. Ravenswaaij-Arts CMAv. Heart rate variability. Ann Intern Med. 1993;118(6):436–447. doi: 10.7326/0003-4819-118-6-199303150-00008. [DOI] [PubMed] [Google Scholar]
- 34.Yasuma F, Hayano J. Respiratory sinus arrhythmia: why does the heartbeat synchronize with respiratory rhythm? Chest. 2004;125(2):683–690. doi: 10.1378/chest.125.2.683. [DOI] [PubMed] [Google Scholar]
- 35.Berntson GG, Cacioppo JT, Quigley KS. Respiratory sinus arrhythmia: autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993;30(2):183–196. doi: 10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- 36.Kazuma N, Otsuka K, Matsuoka I, Murata M. Heart rate variability during 24 hours in asthmatic children. Chronobiol Int. 1997;14(6):597–606. doi: 10.3109/07420529709001450. [DOI] [PubMed] [Google Scholar]
- 37.Kim D-K, Rhee J-H, Kang SW. Reorganization of the brain and heart rhythm during autogenic meditation. Front Integr Nuerosci. 2014;7:109. doi: 10.3389/fnint.2013.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141(2):122–131. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- 39.Valentini M, Parati G. Variables influencing heart rate. Prog Cardiovasc Dis. 2009;52(1):11–19. doi: 10.1016/j.pcad.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Lanfranchi PA, Somers VK. Arterial baroreflex function and cardiovascular variability: interactions and implications. Am J Physiol Regul Integr Comp Physiol. 2002;283(4):R815–R826. doi: 10.1152/ajpregu.00051.2002. [DOI] [PubMed] [Google Scholar]
- 41.Stauss HM. Heart rate variability. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R927–R931. doi: 10.1152/ajpregu.00452.2003. [DOI] [PubMed] [Google Scholar]
- 42.Rennie KL, Hemingway H, Kumari M, Brunner E, Malik M, Marmot M. Effects of moderate and vigorous physical activity on heart rate variability in a British study of civil servants. Am J Epidemiol. 2003;158(2):135–143. doi: 10.1093/aje/kwg120. [DOI] [PubMed] [Google Scholar]
- 43.Serrador JM, Finlayson HC, Hughson RL. Physical activity is a major contributor to the ultra low frequency components of heart rate variability. Heart. 1999;82(6 e9):e9. doi: 10.1136/hrt.82.6.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Routledge FS, Campbell TS, McFetridge-Durdle JA, Bacon SL. Improvements in heart rate variability with exercise therapy. Can J Cardiol. 2010;26(6):303–312. doi: 10.1016/s0828-282x(10)70395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aubert AE, Seps B, Beckers F. Heart rate variability in athletes. Sports Med. 2003;33(12):889–919. doi: 10.2165/00007256-200333120-00003. [DOI] [PubMed] [Google Scholar]
- 46.Thayer JF, Hall M, Sollers JJ, III, Fischer JE. Alcohol use, urinary cortisol, and heart rate variability in apparently healthy men: Evidence for impaired inhibitory control of the HPA axis in heavy drinkers. Int J Psychophysiol. 2006;59(3):244–250. doi: 10.1016/j.ijpsycho.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 47.van Zyl LT, Hasegawa T, Nagata K. The effects of antidepressants on heart rate variability in major depression: a quantitative review. Biopsychosoc Med. 2008;30(2):12–22. doi: 10.1186/1751-0759-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nolan RP, Jong P, Barry-Bianchi SM, Tanaka TH, Floras JS. Effects of drug, biobehavioral and exercise therapies on heart rate variability in coronary artery disease: a systematic review. Eur J Cardiovasc Prev Rehabil. 2008;15(4):386–396. doi: 10.1097/HJR.0b013e3283030a97. [DOI] [PubMed] [Google Scholar]
- 49.Delgado-Pastor LC, Perakakis P, Subramanya P, Telles S, Vila J. Mindfulness (Vipassana) meditation: effects on P3b event-related potential and heart rate variability. Int J Psychophysiol. 2013;90(2):207–214. doi: 10.1016/j.ijpsycho.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 50.Singh JP, Larson MG, O’Donnell CJ, Tsuji H, Evans JC, Levy D. Heritability of heart rate variability: the Framingham Heart Study. Circulation. 1999;99(17):2251–2254. doi: 10.1161/01.cir.99.17.2251. [DOI] [PubMed] [Google Scholar]
- 51.Kupper NHM, Willemsen G, van den Berg M et al. Heritability of ambulatory heart rate variability. Circulation. 2004;110(18):2792–2796. doi: 10.1161/01.CIR.0000146334.96820.6E. [DOI] [PubMed] [Google Scholar]
- 52.Wang X, Thayer JF, Treiber F, Snieder H. Ethnic differences and heritability of heart rate variability in African- and European American youth. Am J Cardiol. 2005;96(8):1166–1172. doi: 10.1016/j.amjcard.2005.06.050. [DOI] [PubMed] [Google Scholar]
- 53.Togo F, Takahashi M. Heart rate variability in occupational health —a systematic review. Ind Health. 2009;47(6):589–602. doi: 10.2486/indhealth.47.589. [DOI] [PubMed] [Google Scholar]
- 54.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 55.Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry. 2010;67(11):1067–1074. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 56.Jeronimus BF, Riese H, Sanderman R, Ormel J. Mutual reinforcement between neuroticism and life experiences: a five-wave, 16-year study to test reciprocal causation. J Pers Soc Psychol. 2014;107(4):751–64. doi: 10.1037/a0037009. [DOI] [PubMed] [Google Scholar]
- 57.Di Simplicio M, Costoloni G, Western D, Hanson B, Taggart P, Harmer CJ. Decreased heart rate variability during emotion regulation in subjects at risk for psychopathology. Psychol Med. 2012;42(8):1775–1783. doi: 10.1017/S0033291711002479. [DOI] [PubMed] [Google Scholar]
- 58.Fredrickson BL, Levenson RW. Positive Emotions Speed Recovery from the Cardiovascular Sequelae of Negative Emotions. Cogn Emotion. 1998;12(2):191–220. doi: 10.1080/026999398379718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de la Torre JC, Torre JCdl. Alzheimer disease as a vascular disorder: nosological evidence. Stroke. 2002;33(4):1152–1162. doi: 10.1161/01.str.0000014421.15948.67. [DOI] [PubMed] [Google Scholar]
- 60.de la Torre JC, Torre JCdl. Is Alzheimer’s disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 2004;3(3):184–190. doi: 10.1016/S1474-4422(04)00683-0. [DOI] [PubMed] [Google Scholar]


