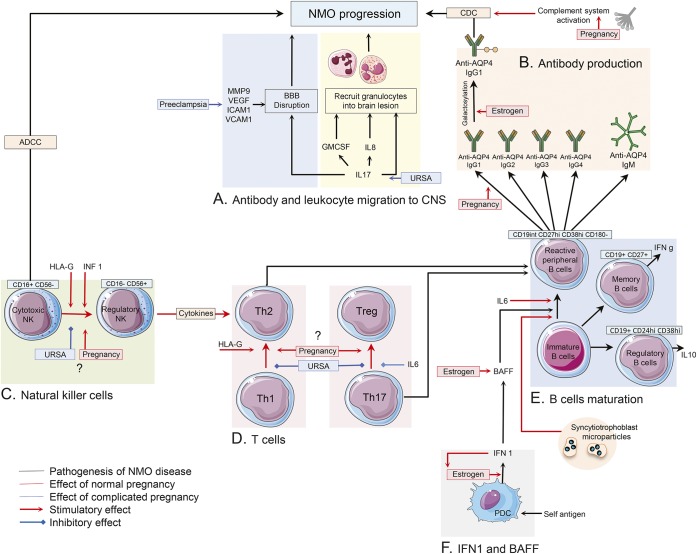
Figure 2. Immune changes in peripheral blood of pregnant patients with NMO.
(A) Interleukin (IL)–17, IL-8, and granulocyte colony-stimulating factor (G-CSF) are proposed as a signal for the recruitment of neutrophils and eosinophil into the perivascular spaces. Matrix metalloproteinase–9 (MMP-9), vascular endothelial growth factor A (VEGF-A), intercellular adhesion molecule–1 (ICAM-1), and vascular cell adhesion molecule–1 (VCAM-1) are considered to have roles in blood–brain barrier (BBB) disruption and lymphocyte recruitment into the CNS of patients with NMO. Serum level of most of these molecules was found to be elevated during preeclampsia. (B) Change in AQP4 antibody classes, subclasses, and glycosylation pattern under influence of sex hormones during and after pregnancy may cause NMO disease progression. (C) During normal pregnancy, NMO could subside due to decreased rate of cytotoxic natural killer cells (NKCs) (CD16+) in peripheral blood, though regulatory NKCs (CD56+) can enhance T-helper (Th2)–mediated immunity and cause NMO disease progression. In patients with unknown recurrent spontaneous abortion (URSA), a shift toward cytotoxic NKCs was reported. Interferon (IFN)–β and human leukocyte antigen G induce a decreased number of cytotoxic NKCs. (D, E) CD19int CD27high CD38high CD180− B-cell plasmablasts are responsible for producing AQP4–immunoglobulin G (IgG) in patients with NMO. IL-6 is important in the survival and activity of these self-reactive peripheral B cells. Increased levels of Th2 immunity, IL-6, B-cell activating factor (BAFF), and IFN 1 in normal pregnancy may cause disease deterioration among patients with NMO, though increased Th17 immunity in complicated pregnancy may have the same effect on NMO progression. Frequency of memory and regulatory B cells and their effects on disease status are required to be investigated in pregnant patients with NMO. (F) Estrogen enhances type 1 IFN responses in plasmacytoid DCs. Type 1 IFNs enhance estrogen signaling in a feed-forward loop. IFN-β treatment worsens the disease course of patients with NMO. Also, IFN-β treatment induces elevated levels of serum BAFF level in patients with NMO. BAFF has a regulatory role in survival and maturation of peripheral B cells. Estrogen boosted BAFF production via stimulation of myeloid cells. ADCC = antibody-dependent cell-mediated cytotoxicity; CDC = complement-dependent cytotoxicity; HLA = human leukocyte antigen; PDC = plasmacytoid dendritic cells.