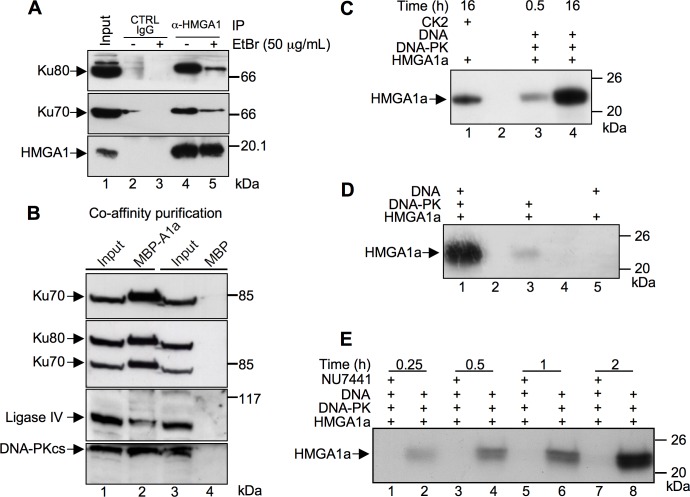
Fig 1. HMGA1a associate with the NHEJ DNA repair protein machinery and is a DNA-PK substrate.
A. Co-immunoprecipitation (Co-IP) assay of endogenous HMGA1, Ku70, and Ku80 proteins on MDA-MB-231 cells in the presence of absence of Ethidium Bromide (EtBr). Cell lysates were immunoprecipitated with α-HMGA1. The cell lysates (input) and immunoprecipitates were analyzed by immunoblotting with antibodies as indicated. B. HMGA1a fused to MBP (MBP-A1a) or the MBP alone were produced by transient transfection in HEK 293T cells. Cellular lysates (input, lanes 1 and 3) were incubated with amylose resin and affinity captured MBP-HMGA1a and MBP proteins recovered. Bound proteins were eluted by SDS sample buffer, separated by SDS-PAGE (T = 10%), and analyzed by western blot using antibodies specific for Ku70, Ku80 (after Ku70 recognition), Ligase IV, and the catalytic subunit of DNA-PK, DNA-PKcs. C. Recombinant HMGA1a protein was subjected to a phosphorylation assay in presence of [γ-32P] ATP with DNA-PK for 0.5 and 16 h (lanes 3 and 4, respectively) and CK2 for 16 h (lane 1). D. Recombinant HMGA1a was subjected to a phosphorylation assay for 16 h with a complete DNA-PK reaction mix (lane 1), without activating DNA (lane 3), or without DNA-PK itself (lane 5). E. Time course phosphorylation assay (0.25, 0.5, 1, and 2 hours) performed with recombinant HMGA1a in the presence (lanes 1, 3, 5, and 7) or absence (lanes 2, 4, 6, and 8) of a specific DNA-PK inhibitor (NU7441–50 nM). Phosphorylated proteins were separated by SDS-PAGE (T = 15%) and 32P incorporation visualized by autoradiography. Protein molecular markers (kDa) are indicated on the right.