Abstract
Prior studies have shown that women have declines in bone structure and strength after hip fracture, but it is unclear whether men sustain similar changes. Therefore, the objective was to examine sex differences in proximal femur geometry following hip fracture. Hip structural analysis was used to derive metrics of bone structure and strength: aerial bone mineral density, cross-sectional bone area (CSA), cortical outer diameter, section modulus (SM), and buckling ratio (BR) from dual-energy x-ray absorptiometry scans performed at baseline (within 22 days of hospital admission), two, six, or twelve months after hip fracture in men and women (n=282) enrolled in the Baltimore Hip Studies 7th cohort. Weighted estimating equations were used to evaluate sex differences at the narrow neck (NN), intertrochanteric (IT), and femoral shaft (FS). Men had significantly different one year NN changes compared to women in CSA: −6.33% (−12.47, −0.20) vs. 1.37% (−3.31, 6.43), P=0.049; SM: −4.98% (−11.08, 1.10) vs. 3.94% (−2.51, 10.42), P=0.042; and BR: 7.50% (0.65, 14.36) vs. −1.20% (−6.41, 4.00), P=0.044. One year IT changes displayed similar patterns, but the sex differences were not statistically significant for CSA: −4.07% (−10.83, 2.67) vs. 0.41% (−3.41, 4.24), P=0.252; SM: −4.78% (-12.10, 5.53) vs. −0.31 (−4.74, 4.11), P=0.287; and BR: 4.59% (−0.65, 9.84) vs. 1.52% (−4.23, 7.28), P=0.425. Differences in FS geometric parameters were even smaller in magnitude and not significantly different by sex. Women generally experienced non-significant increases in bone tissue and strength following hip fracture, while men had structural declines that were statistically greater at the NN region. Reductions in the mechanical strength of the proximal femur after hip fracture could put men at higher risk for subsequent fractures of the contralateral hip.
Keywords: Aging, DXA, Osteoporosis, Injury/Fracture healing, Fracture Prevention
1.0 Introduction
Osteoporosis is a systemic condition characterized by a reduction in the structural strength of bones. The most significant consequence of the disease is often hip fracture, which is associated with excess mortality, morbidity, and health care expenditures [1,2]. Rates of bone fragility and hip fracture are higher in women compared to men, in part, due to the larger skeletal size and bone mass of men and bone loss that occurs in women during menopause [3–5]. Most prior research examining skeletal determinants of hip fracture risk and recovery have focused on bone mineral density, which is a surrogate marker for mechanical strength and not a uniquely distinct geometric property of bone tissue [6–8]. Geometric parameters capture the spatial distribution of bone tissue in ways that are relevant to strength in bending, axial compression, and cortical instability and are independently predictive of hip fracture even after accounting for aerial BMD and other clinical risk factors [9–11]. Geometric properties of bone tissue can be used to understand how changes in bone structure and strength during the natural course of aging contribute to sex differences in the pathogenesis of osteoporosis and hip fracture [1].
Studies of sex differences in bone geometry across adult lifespan have consistently demonstrated that compared to women, men have greater aerial BMD, cross-sectional bone areas (CSA), bone diameters, section moduli (bending strength; SM), and lower buckling ratio (cortical instability; BR) at the narrow neck (NN), intertrochanteric region (IT), and femoral shaft (FS) [1,5,11–16]. Cross-sectional investigations indicate that BMD decreases with age in both men and women but expansions in outer diameter serve to preserve bending strength and compensate for net BMD decline [1,12,15,17]. However, alterations in bone structure and strength among women are accompanied by greater increases in indices of cortical instability compared to men [13]. Moreover, these sexual dimorphisms in bone geometry start earlier in women and continue to occur at greater rates in old-old age at common fracture sites at the NN and IT regions [15,17]. The combination of bone mineral loss, thinning of cortex regions, increases in outer diameter, and consequent susceptibility to local buckling among women during aging contribute to their higher likelihood of hip fracture [9–11].
Also of clinical relevance is an understanding of changes in bone structure and strength that occur after hip fracture because such information is necessary for the design and optimization of treatment strategies to maximize recovery and minimize the risk for subsequent fractures. After hip fracture, women experience profound declines in BMD, particularly at the femoral neck, and these declines are greater than observed in unfractured community-dwelling older women [7,18–20]. Women sustaining hip fracture have comparatively lower BMD not only due to their femurs having less bone (lower CSA) but also because the bone is distributed in a large bone diameter at the contralateral non-fractured hip compared to similar postmenopausal women with osteoporosis [21]. They also experience significantly greater declines in bending strength and increases in cortical instability [21]. Thus, increases in bone fragility may occur rapidly after hip fracture and greatly inflate the risk of a second fracture during the recovery period. As existing research is limited to women, no studies have evaluated sex differences in changes to bone geometry that occur after hip fracture, and such differences may potentially affect recovery in different ways between men and women [22]. Research recently reported that men experience clinically significant greater decrements in BMD after hip fracture than women [23]. The objective of this study was to compare temporal patterns in bone geometry measured in men and women in the year following hip fracture.
2.0 Materials and Methods
2.1 Study Data & Sample
The Baltimore Hip Studies 7th cohort (BHS-7) was an observational study designed to compare the metabolic, physiologic, neuromuscular, functional, and clinical consequences of hip fracture between men and women. Patients hospitalized for hip fracture were recruited from one of eight BHS network hospitals in the Baltimore metropolitan area and included those 65 years or older at the time of hospital admission for hip fracture (ICD-9 codes 820.00–820.9). Men were continuously enrolled into the study; women were frequency matched with men on fracture timing within each hospital, which ensured recruitment of an equal number of men and women and minimized any impact of secular changes in care and hospital practice differences. Exclusion criteria were: pathologic fracture, not community-dwelling at the time of fracture, non-English speaker, being bedbound for 6 months before fracture, residence > 70 miles from the hospital, weight > 300 pounds, no surgery, and hardware in the contralateral hip.
A total of 362 hip fracture patients were enrolled (180 males and 182 females). Five participants did not provide data at the baseline or 2-month follow-up visit and another 18 participants were removed as a result of an IRB-requested post procedure audit (6 participants were subsequently found to be ineligible because they did not meet study inclusion criteria and 12 participants were determined to be ineligible secondary to failures of the informed consent process), leaving a sample of 339 participants. Study visits were conducted at baseline (within 22 days of admission) and at two, six, and twelve months after admission, and included questionnaires and measures of body composition and functional performance. Study protocols were reviewed and approved by the University of Maryland, Baltimore IRB and the review boards of participating hospitals. The analytic sample included 282 participants with at least one dual-energy x-ray absorptiometry (DXA) scan that could be used to derive geometric measures of hip structural strength (Figure 1).
Figure 1.
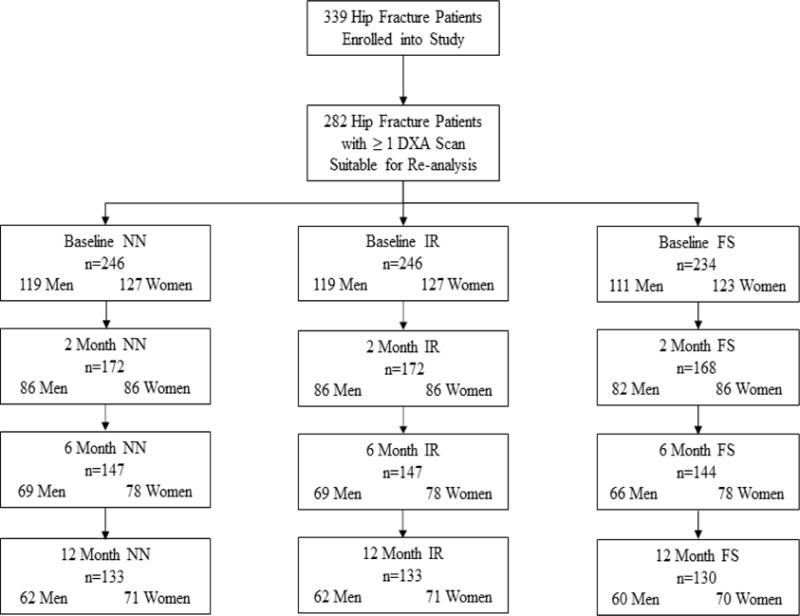
Flow chart for HSA data in the three regions at each time point in the Baltimore Hip Studies 7th cohort.
2.2 DXA Measurement
DXA scans of the total hip were used to evaluate BMD (grams per centimeter squared) longitudinally at the non-fractured hip using either a Lunar Prodigy machine (four sites; Madison, WI, USA) or Hologic machine (three sites; Waltham, MA, USA) at one of seven study DXA facilities. Standardized methods were used for quality control, certification of DXA operators, and scanning procedures to guarantee the reproducibility of results. DXA machines were calibrated daily using a phantom to ensure that no significant measurement drift occurred over time. While it was not always possible to image patents on the same scanner, care was taken to ensure that a scanner from the same manufacturer was consistently used. To account for any inter-machine differences, statistical models included an indicator to capture the type of DXA machine that a given patient was scanned on throughout participation in the study. The estimated sex differences were an average that represented a marginal effect across men and women who used the same type of scanner at each time point.
2.3 Structural Parameters
HSA software used for this study was developed at The Johns Hopkins University and re-analyzes conventional DXA image data to obtain geometric properties of bone cross-sections [8]. Any DXA scans that were judged to be of insufficient quality (e.g., poor image, lack of anatomical coverage) were excluded. The validated HSA algorithm was used to establish three cross-sectional analysis regions on femur DXA images: (1) narrow neck (NN), the narrowest point of the femoral neck; (2) intertrochanteric (IT), along the bisector of the neck-shaft angle; and (3) femoral shaft (FS), a distance of approximately 1.5 times the narrowest neck diameter that is distal to the intersection of neck-shaft axes [24]. The HSA software generates five parallel, pixel mineral mass profiles, along lines that traverse the femur at each of the three regions. From each region: areal BMD (grams per centimeter squared); cross-sectional area (CSA; a measure of bone surface area); outer diameter (OD; a measure of outer cortical width); section modulus (SM; a measure of bending strength); and buckling ratio (BR; a measure of bone instability) were derived from the five profiles and averaged for reporting [8].
2.4 Predictor Variables
All covariates included in multivariable regression models were measured at study enrollment. These variables were selected a priori based on factors that were associated with changes in BMD among women and men in the Study of Osteoporotic Fractures and the Study of Osteoporotic Fractures among Men, respectively [25,26]. Demographic and anthropometric determinants included sex, age (years), race (white or non-white), height (meters), and weight (kilograms). Behavioral factors were smoking (never, past, current) and alcohol consumption (none, minimal, moderate). Clinical characteristics included comorbidity, assessed using a modified version of the Charlson Comorbidity Index that omitted liver disease [27]; depressive symptoms, measured with the Center for Epidemiological Studies Depression Scale [28]; functional limitations, evaluated with the Instrumental Activities of Daily Living (IADL) with a modified version of the Older Americans Resources and Services Instrument [29]. Concomitant medications (never, past, or current) were assessed via patient-reported survey questions and included bone-active drugs, glucocorticoids, hormone therapy, and calcium supplements. Bone-active drugs included Etidronate, Alendronate, Risedronate, Ibandronate, Teriparatide, Calcitonin, Zoledronic Acid, and Pamidronate. Glucocorticoid medications included Prednisone, Cortisone, Hydrocortisone, Dexamethasone, or other steroids. Hormone therapy was dichotomized into binary categories of “ever” and “never” and defined as the use of Estrogen (pill, vaginal cream, suppository, or patch) in women and Testosterone (injection, patch, and gel) in men. Calcium supplements were measured as the daily consumption of Caltrate, Citracal, Os-Cal, or Tums.
2.5 Statistical Analysis
Baseline characteristics were compared between men and women using chi-square tests for categorical covariates and t-tests for continuous variables. Inverse probability of observation weights (IPW) were used to account for missing baseline covariate data, missing longitudinal outcome data, and truncation due to death [30–32]. The weights were the inverse probability of observation given predictors of missing data chosen from the study covariates selected a priori. The IPW approach is used to reweight the analytic sample to be representative of the overall cohort, and more detailed information about the computation and construction of the missing data weights is provided in Appendix A.
Weighted generalized estimating equations (WEE) assuming an independent variance-covariance matrix were fit with robust standard error estimators to account for within-patient clustering of observations. Multivariable models adjusted for the previously described covariates were used to estimate the association between sex and changes in geometric parameters of hip bone strength: aerial BMD, CSA, OD, SM, and BR. Time was modeled as a categorical index to allow for non-linear functional forms. The primary parameter of interest was the interaction between sex and follow-up time that tested whether change from baseline in the geometric structural parameters differed between men and women overall and at each follow-up time point. Multivariable WEE were used to estimate adjusted sex-specific baseline geometric structural parameters, which were calculated holding all covariate values at their sample mean, as well as the corresponding absolute changes and percent changes during follow-up with 95% confidence intervals (95% CI). Statistical significance was set at an alpha level of 0.05 for primary outcome models, and all analyses were conducted using Stata (Version 13, Stata Corp, College Station, Texas, USA).
3.0 Results
3.1 Descriptive Characteristics
The study sample was mostly white with an average age of approximately 80 years (Table 1). Men were significantly heavier and taller than women and were more likely to be smokers and to consume alcohol. Men also had significantly greater levels of comorbidity and functional limitations compared to women. Baseline aerial BMD at the NN, IT, and FS were higher in males and among those participants scanned with a Lunar machine (Supplementary Table 1). Women had a significantly higher likelihood of reporting past or current utilization of bone-active drugs, glucocorticoids, hormone therapy, and calcium supplements. Bisphosphonates, particularly alendronate, were the most frequently reported bone-active drugs that participants disclosed to have ever been taken (Supplementary Table 2).
Table 1.
Baseline characteristics among men and women with HSA data.
| Analytic Sample | |||
|---|---|---|---|
|
| |||
| Variable | Men (n=137) |
Women (n=145) |
P Value |
| White (n, %) | 123 (90.5%) | 134 (93.7%) | 0.32 |
| Age (m, SD) | 80.3 ± 7.7 | 81.0 ± 7.7 | 0.48 |
| Weight (m, SD) | 79.5 ± 14.1 | 63.4 ± 14.3 | <0.0001 |
| Height (m, SD) | 1.8 ± 0.08 | 1.6 ± 0.08 | <0.0001 |
| Smoking (n, %) | |||
| Never | 30 (22.1%) | 67 (46.5%) | <0.0001 |
| Past | 95 (69.9%) | 68 (47.2%) | |
| Present | 11 (8.1%) | 9 (6.3%) | |
| Alcohol Use (n, %) | |||
| None | 53 (40.0%) | 72 (50.0%) | 0.089 |
| Minimal | 79 (58.1%) | 65 (45.1%) | |
| Moderate | 4 (2.9%) | 7 (4.9%) | |
| Bone-Active Drugs (n, %) | |||
| Never | 123 (91.1%) | 84 (58.3%) | <0.0001 |
| Past | 1 (0.7%) | 21 (14.6%) | |
| Current | 11 (8.2%) | 39 (27.1%) | |
| Glucocorticoids | |||
| Never | 106 (80.3%) | 91 (64.1%) | 0.009 |
| Past | 17 (12.9%) | 29 (20.4%) | |
| Current | 9 (6.8%) | 22 (15.5%) | |
| Hormone Therapy | 4 (2.9%) | 54 (37.5%) | <0.0001 |
| Calcium Supplements (n, %) | |||
| Never | 96 (71.1%) | 45 (31.5%) | <0.0001 |
| Past | 14 (10.4%) | 17 (11.9%) | |
| Current | 25 (18.5%) | 81 (56.6%) | |
| Comorbidity (M, IQR) | 2.5 ± 1.9 | 1.7 ± 1.6 | 0.0001 |
| IADL (M, IQR) | 2.2 ± 1.5 | 1.7 ± 1.5 | 0.0099 |
| CES-D (m, SD) | 17.0 ± 9.6 | 17.7 ± 11.2 | 0.55 |
3.2 Narrow Neck (NN)
Men had lower adjusted baseline NN aerial BMD and CSA than women but greater OD, SM, and BR (Table 2). Relative to baseline, aerial BMD and CSA declined in men at the NN from two to twelve months: −0.87% (5.54, 3.78) to −6.36% (−12.81, 0.06) and −1.52% (−6.17, 3.11) to −6.33% (−12.47, −0.20), respectively (Figure 2). In contrast, women experienced non-monotone changes: aerial BMD increased by 0.82% (−2.84, 4.51) at two months but decreased by −0.14% (−4.05, 3.74) at twelve months; and CSA decreased at two months (−0.52%; 95% CI: − 4.01, 2.96) but increased by twelve months (1.37%; 95% CI: −3.31, 6.43). The twelve-month difference in change in NN CSA change (−6.33% vs. 1.37%) was significantly different between men and women (P=0.049), but the global test of the interaction was not statistically significant (P=0.26). There were similar overall changes in OD (P=0.80). However, men experienced greater reductions in SM and increases in BR compared to women. Men had an average decrease in SM of −2.48% (−7.63, 2.64) at two months, −3.76% (−9.64, 2.10) at six months, and −4.98% (−11.08, 1.10) at twelve months. Average twelve month SM change in women (3.94%; 95% CI: −2.51, 10.42) was statistically different compared to men (P=0.042). The twelve-month increase in BR among men of 7.50% (0.65, 14.36) was also significantly different (P=0.044) than the observed decline of −1.20% (−6.41, 4.00) in women. The overall sex differences for SM (P=0.245) and BR (P=0.176) did not reach statistical significance.
Table 2.
Adjusted baseline estimates and relative mean percent change at two, six, and twelve months follow-up in hip structural analysis narrow neck geometric parameters.
| Time Point | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Aerial BMD | % Change | 95% CI | * P | % Change | 95% CI | * P | ** P | *** P |
| Baseline | 0.62 | (0.59, 0.66) | REF | 0.67 | (0.64, 0.70) | REF | 0.109 | 0.418 |
| Δ T2 | −0.87 | (−5.54, 3.78) | 0.71 | 0.82 | (−2.84, 4.51) | 0.655 | 0.559 | |
| Δ T6 | −0.90 | (−6.40, 4.55) | 0.742 | 0.60 | (−2.85, 4.10) | 0.728 | 0.643 | |
| Δ T12 | −6.36 | (−12.81, 0.06) | 0.052 | −0.14 | (−4.05, 3.74) | 0.939 | 0.11 | |
|
| ||||||||
| CSA | ||||||||
|
| ||||||||
| Baseline | 2.11 | (1.98, 2.25) | REF | 2.13 | (1.98, 2.25) | REF | 0.882 | 0.265 |
| Δ T2 | −1.52 | (−6.17, 3.11) | 0.518 | −0.52 | (−4.01, 2.96) | 0.768 | 0.739 | |
| Δ T6 | −2.47 | (−7.75, 2.80) | 0.357 | −0.14 | (−3.95, 3.66) | 0.941 | 0.491 | |
| Δ T12 | −6.33 | (−12.47, −0.20) | 0.043 | 1.37 | (−3.31, 6.43) | 0.565 | 0.049 | |
|
| ||||||||
| OD | ||||||||
|
| ||||||||
| Baseline | 3.53 | (3.45, 3.61) | REF | 3.30 | (3.21, 3.39) | REF | 0.004 | 0.807 |
| Δ T2 | −0.88 | (−2.44, 0.66) | 0.261 | −1.42 | (−2.92, 0.07) | 0.063 | 0.683 | |
| Δ T6 | −1.46 | (−3.25, 0.32) | 0.109 | −1.03 | (−2.76, 0.69) | 0.239 | 0.694 | |
| Δ T12 | 0.52 | (−1.32, 2.38) | 0.575 | 1.35 | (−1.38, 4.08) | 0.332 | 0.649 | |
|
| ||||||||
| SM | ||||||||
|
| ||||||||
| Baseline | 1.22 | (1.13, 1.31) | REF | 1.09 | (1.02, 1.17) | REF | 0.087 | 0.245 |
| Δ T2 | −2.48 | (−7.63, 2.64) | 0.341 | −1.27 | (−5.14, 2.58) | 0.515 | 0.678 | |
| Δ T6 | −3.76 | (−9.64, 2.10) | 0.207 | −0.53 | (−5.03, 3.95) | 0.813 | 0.371 | |
| Δ T12 | −4.98 | (−11.08, 1.10) | 0.108 | 3.94 | (−2.51, 10.42) | 0.23 | 0.042 | |
|
| ||||||||
| BR | ||||||||
|
| ||||||||
| Baseline | 17.34 | (16.31, 18.37) | REF | 15.27 | (14.40, 16.15) | REF | 0.012 | 0.176 |
| Δ T2 | −1.20 | (−6.22, 3.81) | 0.636 | −0.32 | (−6.04, 5.38) | 0.909 | 0.795 | |
| Δ T6 | −0.47 | (−6.87, 5.92) | 0.884 | −2.74 | (−6.96, 1.46) | 0.2 | 0.609 | |
| Δ T12 | 7.50 | (0.65, 14.36) | 0.032 | −1.20 | (−6.41, 4.00) | 0.65 | 0.044 | |
BMD: Bone Mineral Density; CSA: Cross Sectional Area; SM: Section Modulus; BR: Buckling Ratio.
P value for sex- and time-specific changes;
P values for the time-specific differences in change;
P value for the global test of the sex by time interaction.
Figure 2.
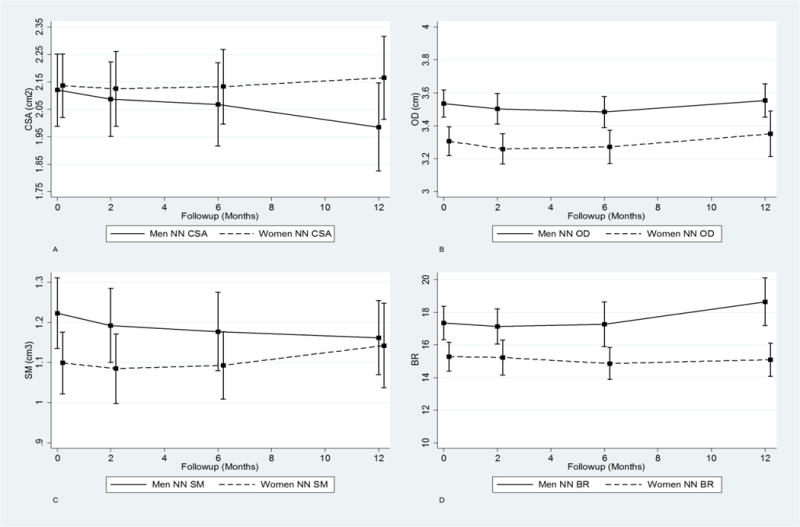
Trajectory plots of adjusted absolute values for the one-year post fracture recovery period for NN CSA (A), OD (B), SM (C), and BR (D).
3.3 Intertrochanteric (IT)
Adjusted baseline IT aerial BMD, CSA, OD, SM, and BR were greater in men than women (Table 3). Estimated geometric parameters at the IT in men displayed a non-linear pattern of change, specifically, decreases at two months, which were attenuated by six months, but eventually reoccurred in greater magnitude at twelve months (Supplementary Figure 1). These patterns are illustrated by changes in CSA in men: −2.66% (−7.90, 2.56) at two months, 0.61% (−5.15, 6.39) at six months, and −4.07% (−10.83, 2.67) at twelve month; and estimates of SM: −4.32% (−10.49, 1.83) at two months, 1.14% (−5.37, 7.66) at six months, and −4.78% at twelve months (−12.10, 5.53). There were similar trends for IT aerial BMD, OD, and BR. Changes in geometric parameters at the IT in women, with the exception of SM, were largest in magnitude at two months but smaller by twelve months. For instance, aerial BMD increased by 2.63% (−1.58, 6.89) at two months; however, at twelve months, the improvement was minimal (0.43%; 95% CI: −3.73, 4.61). Similarly, OD decreased by −0.85% (−1.94, 0.23) at two months, but this change decreased in magnitude to −0.02% by twelve months. IT CSA and BR displayed comparable longitudinal trends. Average percent change in SM was 0.61 % (−3.13, 4.36) at two, −2.89% (−7.07, 1.28) at six, and −0.31% (−4.75, 4.11) at twelve months; this trajectory of change was significantly different compared to men (P=0.048). None of the other time-specific differences or global sex by time interactions were statistically significant.
Table 3.
Adjusted baseline estimates and relative mean percent change at two, six, and twelve months follow-up in hip structural analysis intertrochanteric region geometric parameters.
| Time Point | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Aerial BMD | % Change | 95% CI | * P | % Change | 95% CI | * P | ** P | *** P |
| Baseline | 0.66 | (0.62, 0.70) | REF | 0.64 | (0.61, 0.68) | REF | 0.668 | 0.382 |
| Δ T2 | −1.26 | (−6.22, 3.67) | 0.613 | 2.63 | (−1.58, 6.89) | 0.221 | 0.235 | |
| Δ T6 | 0.71 | (−5.04, 6.48) | 0.807 | 0.86 | (−2.96, 4.69) | 0.658 | 0.971 | |
| Δ T12 | −4.23 | (−10.94, 2.44) | 0.214 | 0.43 | (−3.73, 4.61) | 0.836 | 0.246 | |
|
| ||||||||
| CSA | ||||||||
|
| ||||||||
| Baseline | 3.80 | (3.56, 4.03) | REF | 3.5193 | (3.33, 3.70) | REF | 0.11 | 0.232 |
| Δ T2 | −2.66 | (−7.90, 2.56) | 0.317 | 1.89 | (−1.84, 5.63) | 0.319 | 0.164 | |
| Δ T6 | 0.61 | (−5.15, 6.39) | 0.834 | 0.35 | (−3.25, 5.63) | 0.847 | 0.933 | |
| Δ T12 | −4.07 | (−10.83, 2.67) | 0.236 | 0.41 | (−3.41, 4.24) | 0.831 | 0.252 | |
|
| ||||||||
| OD | ||||||||
|
| ||||||||
| Baseline | 5.99 | (5.88, 6.10) | REF | 5.69 | (5.59, 5.78) | REF | 0.002 | 0.775 |
| Δ T2 | 1.22 | (−2.66, 0.20) | 0.092 | −0.85 | (−1.94, 0.23) | 0.124 | 0.64 | |
| Δ T6 | 0.09 | (−1.47, 1.66) | 0.906 | −0.41 | (−1.70, 0.88) | 0.532 | 0.628 | |
| Δ T12 | 0.56 | (−0.83, 1.96) | 0.429 | −0.02 | (−1.48, 1.44) | 0.976 | 0.562 | |
|
| ||||||||
| SM | ||||||||
|
| ||||||||
| Baseline | 3.91 | (3.64, 4.19) | REF | 3.28 | (3.10, 3.46) | REF | 0.001 | 0.048 |
| Δ T2 | −4.32 | (−10.49, 1.83) | 0.168 | 0.61 | (−3.13, 4.36) | 0.745 | 0.162 | |
| Δ T6 | 1.14 | (−5.37, 7.66) | 0.730 | −2.89 | (−7.07, 1.28) | 0.174 | 0.342 | |
| Δ T12 | −4.78 | (−12.10, 5.53) | 0.199 | −0.31 | (−4.75, 4.11) | 0.888 | 0.287 | |
|
| ||||||||
| BR | ||||||||
|
| ||||||||
| Baseline | 13.47 | (12.59, 14.34) | REF | 13.18 | (12.38, 13.99) | REF | 0.703 | 0.571 |
| Δ T2 | 0.86 | (−3.54, 5.27) | 0.70 | 2.51 | (−4.40, 9.44) | 0.474 | 0.689 | |
| Δ T6 | −0.67 | (−5.90, 4.54) | 0.799 | 1.85 | (−3.27, 6.97) | 0.477 | 0.494 | |
| Δ T12 | 4.59 | (−0.65, 9.84) | 0.086 | 1.52 | (−4.23, 7.28) | 0.602 | 0.425 | |
BMD: Bone Mineral Density; CSA: Cross Sectional Area; SM: Section Modulus; BR: Buckling Ratio.
P value for sex- and time-specific changes;
P values for the time-specific differences in change;
P value for the global test of the sex by time interaction.
3.4 Femoral Shaft (FS)
Men had significantly greater baseline FS aerial BMD, CSA, cortical width, and SM compared to women (Table 4). Men experienced non-linear changes for all FS geometric parameters, where the average percent changes generally declined from baseline to two months, increased from baseline to six months, and decreased again by twelve months (Supplementary Figure 2). For example, estimated percent changes in FS CSA and SM at two, six months, and twelve months were: −0.94% (−5.23, 3.33), 2.28% (−2.97, 7.54), and 0.13% (−5.38, 5.66); and −1.53% (−6.07, 3.00), 1.28% (−4.30, 6.88), and 0.37% (−5.33, 6.09), respectively. Analogous patterns of change were observed for aerial BMD, OD, and BR. The trajectories of change for FS geometric parameters in women were more stable and showed less longitudinal variation. Average percent change in FS CSA was 2.19% (−1.51, 5.90) at two months, 1.70% (−2.26, 5.69) at six months, and 2.48% (−2.29, 7.27) at twelve months. Likewise, estimates of percent change in FS SM were 2.71% (−1.51, 6.94), 1.32% (−2.72, 5.37), and 2.64% (−1.85, 7.15). Aerial BMD, cortical width, and BR showed comparable prospective trends. Neither the time-specific differences nor the global interactions for any of the geometric parameters at the FN were statistically significant.
Table 4.
Adjusted baseline estimates and relative mean percent change at two, six, and twelve months follow-up in hip structural analysis femoral shaft geometric parameters.
| Time Point | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Aerial BMD | % Change | 95% CI | * P | % Change | 95% CI | * P | ** P | *** P |
| Baseline | 1.27 | (1.19, 1.34) | REF | 1.12 | (1.05, 1.19) | REF | 0.025 | 0.813 |
| Δ T2 | −0.72 | (−5.12, 3.64) | 0.741 | 1.27 | (−2.75, 5.31) | 0.533 | 0.514 | |
| Δ T6 | 2.27 | (−2.91, 7.47) | 0.388 | 1.31 | (−2.71, 5.35) | 0.521 | 0.727 | |
| Δ T12 | 0.21 | (−5.46, 5.90) | 0.939 | 1.17 | (−3.92, 6.29) | 0.649 | 0.822 | |
|
| ||||||||
| CSA | ||||||||
|
| ||||||||
| Baseline | 3.87 | (3.64, 4.10) | REF | 3.33 | (3.12, 3.53) | REF | 0.005 | 0.526 |
| Δ T2 | −0.94 | (−5.23, 3.33) | 0.662 | 2.19 | (−1.51, 5.90) | 0.245 | 0.299 | |
| Δ T6 | 2.28 | (−2.97, 7.54) | 0.394 | 1.70 | (−2.26, 5.69) | 0.398 | 0.798 | |
| Δ T12 | 0.13 | (−5.38, 5.66) | 0.96 | 2.48 | (−2.29, 7.27) | 0.307 | 0.568 | |
|
| ||||||||
| OD | ||||||||
|
| ||||||||
| Baseline | 3.19 | (3.13, 3.24) | REF | 3.07 | (3.02, 3.13) | REF | 0.019 | 0.406 |
| Δ T2 | −0.40 | (−1.77, 0.96) | 0.557 | 1.96 | (−1.48, 5.41) | 0.263 | 0.208 | |
| Δ T6 | −0.07 | (−1.49, 1.33) | 0.912 | 0.76 | (−0.51, 2.04) | 0.242 | 0.406 | |
| Δ T12 | 0.12 | (−1.61, 1.87) | 0.883 | 1.85 | (0.29, 3.42) | 0.02 | 0.167 | |
|
| ||||||||
| SM | ||||||||
|
| ||||||||
| Baseline | 2.35 | (2.21, 2.49) | REF | 1.99 | (1.87, 2.10) | REF | 0.002 | 0.447 |
| Δ T2 | −1.53 | (−6.07, 3.00) | 0.507 | 2.71 | (−1.51, 0.694) | 0.208 | 0.196 | |
| Δ T6 | 1.28 | (−4.30, 6.88) | 0.651 | 1.32 | (−2.72, 5.37) | 0.52 | 0.96 | |
| Δ T12 | 0.37 | (−5.33, 6.09) | 0.896 | 2.64 | (−1.85, 7.15) | 0.248 | 0.596 | |
|
| ||||||||
| BR | ||||||||
|
| ||||||||
| Baseline | 3.85 | (3.52, 4.18) | REF | 4.38 | (4.05, 4.72) | REF | 0.076 | 0.713 |
| Δ T2 | −0.18 | (−6.14, 5.76) | 0.95 | 6.57 | (−6.24, 19.39) | 0.313 | 0.336 | |
| Δ T6 | −1.00 | (−7.45, 5.45) | 0.76 | −2.17 | (−7.68, 3.34) | 0.439 | 0.741 | |
| Δ T12 | 0.88 | (−6.39, 8.18) | 0.81 | 2.68 | (−4.46, 9.83) | 0.461 | 0.695 | |
BMD: Bone Mineral Density; CSA: Cross Sectional Area; SM: Section Modulus; BR: Buckling Ratio.
P value for sex- and time-specific changes;
P values for the time-specific differences in change;
P value for the global test of the sex by time interaction.
4.0 Discussion
The results showed that men had significantly different patterns of change in proximal femur structural strength compared to women after hip fracture. Women in BHS-7 generally experienced negligible changes with respect to the spatial distribution of bone tissue influencing structural strength. By contrast, men showed declines in CSA, SM, and increases in BR, particularly at the NN, where the estimated twelve-month differences in change reached statistical significance. In context, normal aging among women is associated with greater aerial BMD loss and increases to cortical outer diameter compared to men, as well as greater corresponding declines in bending strength and increases in susceptibility to local buckling [1,12,15,17]. However, our results imply that in the year following hip fracture the structural advantage of males may be reversed.
The findings from the current study suggest that women had non-significant increases in bone tissue, and in some instances, these changes to the spatial distribution of bone mineral were accompanied by minimal increases in bending strength and cortical stability. Previous BHS research has shown that women experience significant declines in BMD at the intertrochanteric region and femoral neck after hip fracture that are greater compared to community-dwelling older women [7,18–20]. Further, one study found decreases in cross-sectional bone area, bending strength, and cortical stability among women following hip fracture that were significantly greater compared to similar postmenopausal women with osteoporosis [21]. Current guidelines recommend long-term bisphosphonate treatment for osteoporosis, and bone-active drug use after hip fracture has increased since research has demonstrated decreases in the risk of subsequent fracture, mortality, and further BMD loss [33–35]. Prior BHS research used data from earlier cohorts, for example, the BHS 3rd cohort who were recruited from 1992 to 1995 [21]. During the BHS-7 recruitment time period from 2006 to 2010, prescriptions of bone-active drugs in the US increased from 21 million in 2002 to 31 million in 2007, and hip fracture incidence among American women decreased over this same interval through 2012 [36,37]. Thus, more favorable bone geometry outcomes among women in BHS-7 may reflect improvements in osteoporosis management prior to and after hip fracture. Cohort effects and differences in study inclusion and exclusion criteria could also contribute to the divergence in results of the current study compared to previous research [21,23]. Specficially, differences in cohort characteristics or patient case mix may account for the observed findings because women experiencing hip fractures in the 21st century could have had different pre-fracture lifetime exposures compared to women who fractured when earlier BHS studies were conducted.
Men had significantly greater twelve-month decreases at the NN in the amount of cross-sectional bone area and declines in bending strength and increases in cortical instability that were significantly different compared to changes in women. Despite decreases in BMD over time, men have stable NN SM throughout life course, a result of enlargements to outer diameter that offset these decrements in the amount of bone tissue [1,12]. Unlike men, compensatory changes in bone structure among women that maintain bending strength occur in conjunction with significant increases in BR [13,17]. However, the findings of this study illustrate a different pattern of change in bone geometry in men who have sustained hip fracture compared to the changes associated with normal aging. The results of the current study are congruent with data suggesting greater post-fracture declines in total hip and femoral neck BMD in men compared to women and more closely resembles the documented patterns in bone geometry among women that occur in the context of aging [23]. The finding that these changes occurred disproportionately at the narrow neck are consistent with previous research indicating that long bone ends sustain more age-related BMD loss [38]. Aging predominantly impacts men at the neck region in the context of bone structure and strength, whereas women are affected at both the neck and intertrochanteric region, and low impact fractures at the NN are most common under the lowest structural mechanical strength levels [15,39]. Changes to bone geometry may put men at higher risk for subsequent fracture of the neck region at the contralateral hip, but what remains unclear from this study are the mediating mechanisms responsible for the greater losses in bone tissue, bending strength, and cortical stability.
Several factors may contribute to sex differences in hip bone geometry following hip fracture. Men have age-related accelerations in BMD decline later in life, while women experience attenuations in decline that may be due to prior decrements that start after menopause, and this divergence may affect the spatial distribution of bone tissue [23,40–42]. There also are hormonal changes in factors known to influence bone mineralization that occur post-fracture, such as Insulin-like growth hormone-1, parathyroid hormone, and osteocalcin, which could differentially affect bone formation and structure between men and women [22,43–45]. Further, osteoporosis is under-recognized in men and does not receive the same level of clinical attention [46]. Men are 10–20 times more likely to be under-treated with bisphosphonates and have lower rates of DXA referral and osteoporosis treatment initiation after hip fracture [47,48]. Disproportionately better osteoporosis care prior to and after hip fracture may lead to differences in musculoskeletal outcomes. Likewise, femoral bone structure and strength adapts to skeletal loading; that is, increases in weight loss and declines in physical performance are associated with poorer mechanical homeostasis [14,49,50]. Men are also generally in poorer health at the time of fracture (i.e., greater comorbidity), and if frailty following hip fracture occurs more frequently in men, physiological changes and a deconditioning of the body could result in losses to bone mass and strength. The pathways leading to sex differences in hip bone geometry are likely heterogeneous and comprised of a multitude of mechanisms.
There are limitations that should be considered when interpreting the results. Two types of DXA scanners were used, and this could have influenced the magnitude of the estimated percent changes within-sex. However, male and female hip fracture patients were matched on clinical site and thus DXA manufacturer; longitudinal sex differences were therefore independent of machine effects. The HSA method for measuring femur geometry has a number of intrinsic limitations, which have been described previously in detail elsewhere, and they are mainly a consequence of the two-dimensional nature of DXA images [24]. Femurs are three-dimensional objects, but the dimensions and the distribution of the mineral mass in the DXA scan depend strongly on how the femur is positioned in the projected image. Positioning uncertainties require larger samples and longer observation periods to detect subtle differences or changes in hip bone structure. The buckling ratio is, at best, a crude index of cortical instability based on an estimate of the average cortical thickness. It is well recognized that the femoral neck cortex is highly asymmetric and favors the physiologically loaded medial surface [51]. Use of the average cortex probably underestimates the contribution of local buckling to failure in a fall to the side, however, failure mechanisms cannot be reliably predicted from the rudimentary structural information present in a DXA scan. Concomitant medication use data was captured using self-reported questionnaires and information on compliance and adherence was not collected. The BHS-7 cohort primarily recruited white men and women from Baltimore metropolitan area and may lack generalizability to more ethnically and regionally diverse hip fracture samples. Last, there is the potential for confounding by unmeasured factors not included in the analysis.
Nonetheless, the various strengths of the study mitigate any potential sources of bias. BHS-7 is one of the largest cohorts of men and women hip fracture patients that was specifically designed to examine sex differences in the sequelae of hip fracture. Thus, comprehensive and relevant information was collected from clinical and patient-reported measures on a multitude of important confounders: body size and mass, comorbidity, physical function, and bone-active medications. The analytical approach also utilized methods to account for missing covariate and outcome data and truncation due to death. Our research is the first to evaluate sex differences in bone geometry after hip fracture and has yielded new information on how patterns of change in bone structure and strength differ between men and women.
5.0 Conclusions
The findings from the current study build upon previous research. First, women were shown to have minimal increases in bone structure and strength that were not significantly different from zero. Second, men experienced declines in the spatial distribution of bone tissue and reductions in mechanical bone strength at the NN region that were significantly different compared to women. The evidence indicates that men may be at greater subsequent risk for low impact fractures of the contralateral non-fractured hip and underscores the necessity for better clinical management of osteoporosis among men prior to and after hip fracture in order to improve post-fracture musculoskeletal outcomes. Future research should ascertain how changes in hip bone geometry in men after hip fracture compare to the normal variation associated with aging and also attempt to identify the mediating mechanisms responsible for the observed sex differences in bone structure and strength.
Supplementary Material
Highlights.
Women generally showed non-significant increases in bone tissue, bending strength, and cortical stability following hip fracture
Men experienced statistically greater one-year declines in cross-sectional bone area, bending strength, and cortical stability than women after hip fracture
Men may be at increased risk for subsequent fracture of the non-fractured contralateral after incident hip fracture
Findings underscore the need for better osteoporosis care among men prior to and after hip fracture
Acknowledgments
This research was supported by grants from the National Institute on Aging (R37 AG009901, R01 AG029315, P30 AG028747, and T32 AG00262). The funding sponsor had no role in study design; in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the article for publication. We also acknowledge the cooperation of the hospitals, DXA facilities, residents, and families participating in this project.
Abbreviations
- HSA
hip structural analysis
- BMD
bone mineral density
- CSA
cross-sectional area
- OD
outer diameter
- SM
section modulus
- BR
buckling ratio
- NN
narrow neck
- IT
intertrochanteric region
- FS
femoral shaft
- BHS
Baltimore hip studies
- DXA
dual-energy x-ray absorptiometry
- IPW
inverse probability of observation weights
- WEE
weighted generalized estimating equations
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
Dr. Jay Magaziner has consulting agreements with Ammonett, Novartis, Sanofi, and Viking. Dr. Denise Orwig has consulting agreements with Kinexum and Viking. Dr. Thomas J. Beck is CEO of Beck Radiological Innovations Inc. Drs. Alan M. Rathbun, Michelle Shardell, J. Richard Hebel, Gregory E. Hicks, and Marc C. Hochberg have no conflicts of interest to declare.
References
- 1.Beck TJ, Ruff CB, Scott WW, Jr, Plato CC, Tobin JD, Quan CA. Sex differences in geometry of the femoral neck with aging: a structural analysis of bone mineral data. Calcif Tissue Int. 1992;50(1):24–29. doi: 10.1007/BF00297293. [DOI] [PubMed] [Google Scholar]
- 2.Melton L. Hip fractures: a worldwide problem today and tomorrow. Bone. 1993;14:1–8. doi: 10.1016/8756-3282(93)90341-7. [DOI] [PubMed] [Google Scholar]
- 3.Seeman E. Pathogenesis of bone fragility in women and men. The Lancet. 2002;359(9320):1841–1850. doi: 10.1016/S0140-6736(02)08706-8. [DOI] [PubMed] [Google Scholar]
- 4.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. The Lancet. 2002;359(9319):1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 5.Looker AC, Beck TJ, Orwoll ES. Does body size account for gender differences in femur bone density and geometry? Journal of Bone and Mineral Research. 2001;16(7):1291–1299. doi: 10.1359/jbmr.2001.16.7.1291. [DOI] [PubMed] [Google Scholar]
- 6.Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, et al. Predictive value of BMD for hip and other fractures. Journal of bone and mineral research. 2005;20(7):1185–1194. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 7.Magaziner J, Wehren L, Hawkes W, Orwig D, Hebel J, Fredman L, et al. Women with hip fracture have a greater rate of decline in bone mineral density than expected: another significant consequence of a common geriatric problem. Osteoporosis Int. 2006;17(7):971–977. doi: 10.1007/s00198-006-0092-3. [DOI] [PubMed] [Google Scholar]
- 8.Beck TJ. Extending DXA beyond bone mineral density: understanding hip structure analysis. Current osteoporosis reports. 2007;5(2):49–55. doi: 10.1007/s11914-007-0002-4. [DOI] [PubMed] [Google Scholar]
- 9.Rivadeneira F, Zillikens MC, De Laet CE, Hofman A, Uitterlinden AG, Beck TJ, et al. Femoral neck BMD is a strong predictor of hip fracture susceptibility in elderly men and women because it detects cortical bone instability: the Rotterdam Study. Journal of Bone and Mineral Research. 2007;22(11):1781–1790. doi: 10.1359/jbmr.070712. [DOI] [PubMed] [Google Scholar]
- 10.Kaptoge S, Beck TJ, Reeve J, Stone KL, Hillier TA, Cauley JA, et al. Prediction of incident hip fracture risk by femur geometry variables measured by hip structural analysis in the study of osteoporotic fractures. Journal of Bone and Mineral Research. 2008;23(12):1892–1904. doi: 10.1359/JBMR.080802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LaCroix AZ, Beck TJ, Cauley JA, Lewis CE, Bassford T, Jackson R, et al. Hip structural geometry and incidence of hip fracture in postmenopausal women: what does it add to conventional bone mineral density? Osteoporosis Int. 2010;21(6):919–929. doi: 10.1007/s00198-009-1056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beck TJ, Looker AC, Ruff CB, Sievanen H, Wahner HW. Structural trends in the aging femoral neck and proximal shaft: analysis of the Third National Health and Nutrition Examination Survey dual-energy X-ray absorptiometry data. Journal of Bone and Mineral Research. 2000;15(12):2297–2304. doi: 10.1359/jbmr.2000.15.12.2297. [DOI] [PubMed] [Google Scholar]
- 13.Kaptoge S, Dalzell N, Loveridge N, Beck TJ, Khaw K, Reeve J. Effects of gender, anthropometric variables, and aging on the evolution of hip strength in men and women aged over 65. Bone. 2003;32(5):561–570. doi: 10.1016/s8756-3282(03)00055-3. [DOI] [PubMed] [Google Scholar]
- 14.Kaptoge S, Dalzell N, Jakes R, Wareham N, Day N, Khaw K, et al. Hip section modulus, a measure of bending resistance, is more strongly related to reported physical activity than BMD. Osteoporosis Int. 2003;14(11):941–949. doi: 10.1007/s00198-003-1484-2. [DOI] [PubMed] [Google Scholar]
- 15.Djonic D, Milovanovic P, Nikolic S, Ivovic M, Marinkovic J, Beck T, et al. Inter-sex differences in structural properties of aging femora: implications on differential bone fragility: a cadaver study. J Bone Miner Metab. 2011;29(4):449–457. doi: 10.1007/s00774-010-0240-x. [DOI] [PubMed] [Google Scholar]
- 16.Litwic A, Clynes M, Denison H, Jameson K, Edwards M, Sayer A, et al. Non-invasive Assessment of Lower Limb Geometry and Strength Using Hip Structural Analysis and Peripheral Quantitative Computed Tomography: A Population-Based Comparison. Calcif Tissue Int. 2015:1–7. doi: 10.1007/s00223-015-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yates LB, Karasik D, Beck TJ, Cupples LA, Kiel DP. Hip structural geometry in old and old-old age: similarities and differences between men and women. Bone. 2007;41(4):722–732. doi: 10.1016/j.bone.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox K, Magaziner J, Hawkes W, Yu-Yahiro J, Hebel J, Zimmerman S, et al. Loss of bone density and lean body mass after hip fracture. Osteoporosis Int. 2000;11(1):31–35. doi: 10.1007/s001980050003. [DOI] [PubMed] [Google Scholar]
- 19.Wehren LE, Hawkes WG, Hebel JR, Orwig D, Zimmerman SI, Fox KM, et al. Predictors of bone loss after hip fracture. Osteoporosis Int. 2004;15(2):125–131. doi: 10.1007/s00198-003-1498-9. [DOI] [PubMed] [Google Scholar]
- 20.Wehren LE, Hawkes WG, Hebel JR, Orwig DL, Magaziner J. Bone mineral density, soft tissue body composition, strength, and functioning after hip fracture. J Gerontol A Biol Sci Med Sci. 2005 Jan;60(1):80–84. doi: 10.1093/gerona/60.1.80. [DOI] [PubMed] [Google Scholar]
- 21.Reider L, Beck T, Hochberg M, Hawkes W, Orwig D, YuYahiro J, et al. Women with hip fracture experience greater loss of geometric strength in the contralateral hip during the year following fracture than age-matched controls. Osteoporosis Int. 2010;21(5):741–750. doi: 10.1007/s00198-009-1000-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orwig DL, Chan J, Magaziner J. Hip fracture and its consequences: differences between men and women. Orthop Clin North Am. 2006;37(4):611–622. doi: 10.1016/j.ocl.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Rathbun AM, Shardell M, Orwig D, Hebel JR, Hicks GE, Beck T, et al. Differences in the trajectory of bone mineral density change measured at the total hip and femoral neck between men and women following hip fracture. Archives of Osteoporosis. 2016;11(1):1–9. doi: 10.1007/s11657-016-0263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beck TJ, Broy SB. Measurement of Hip Geometry—Technical Background. Journal of Clinical Densitometry. 2015;18(3):331–337. doi: 10.1016/j.jocd.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Cummings SR, Black DM, Nevitt MC, Browner WS, Cauley JA, Genant HK, et al. Appendicular bone density and age predict hip fracture in women. JAMA. 1990;263(5):665–668. [PubMed] [Google Scholar]
- 26.Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemporary clinical trials. 2005;26(5):569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Charlson ME, Charlson RE, Peterson JC, Marinopoulos SS, Briggs WM, Hollenberg JP. The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol. 2008 Dec;61(12):1234–1240. doi: 10.1016/j.jclinepi.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Applied psychological measurement. 1977;1(3):385–401. [Google Scholar]
- 29.Plotnikoff RC, Brez S, Hotz SB. Exercise behavior in a community sample with diabetes: understanding the determinants of exercise behavioral change. Diabetes Educ. 2000 May-Jun;26(3):450–459. doi: 10.1177/014572170002600312. [DOI] [PubMed] [Google Scholar]
- 30.Robins JM, Rotnitzky A, Zhao LP. Estimation of regression coefficients when some regressors are not always observed. Journal of the American Statistical Association. 1994;89(427):846–866. [Google Scholar]
- 31.Robins JM, Rotnitzky A, Zhao LP. Analysis of semiparametric regression models for repeated outcomes in the presence of missing data. Journal of the American Statistical Association. 1995;90(429):106–121. [Google Scholar]
- 32.Shardell M, Hicks GE, Miller RR, Magaziner J. Semiparametric regression models for repeated measures of mortal cohorts with non-monotone missing outcomes and time-dependent covariates. Stat Med. 2010;29(22):2282–2296. doi: 10.1002/sim.3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adler RA, Fuleihan GE, Bauer DC, Camacho PM, Clarke BL, Clines GA, et al. Managing Osteoporosis in Patients on Long-Term Bisphosphonate Treatment: Report of a Task Force of the American Society for Bone and Mineral Research. Journal of Bone and Mineral Research. 2016;31(1):16–35. doi: 10.1002/jbmr.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lyles KW, Colón-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799–1809. doi: 10.1056/NEJMoa074941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magaziner JS, Orwig DL, Lyles KW, Nordsletten L, Boonen S, Adachi JD, et al. Subgroup variations in bone mineral density response to zoledronic acid after hip fracture. Journal of Bone and Mineral Research. 2014;29(12):2545–2551. doi: 10.1002/jbmr.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wysowski DK, Greene P. Trends in osteoporosis treatment with oral and intravenous bisphosphonates in the United States, 2002–2012. Bone. 2013;57(2):423–428. doi: 10.1016/j.bone.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Stevens J, Rudd R. The impact of decreasing US hip fracture rates on future hip fracture estimates. Osteoporosis Int. 2013;24(10):2725–2728. doi: 10.1007/s00198-013-2375-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sievänen H, Uusi-Rasi K, Heinonen A, Oja P, Vuori I. Disproportionate, age-related bone loss in long bone ends: a structural analysis based on dual-energy X-ray absorptiometry. Osteoporosis Int. 1999;10(4):295–302. doi: 10.1007/s001980050230. [DOI] [PubMed] [Google Scholar]
- 39.Pulkkinen P, Eckstein F, Lochmüller E, Kuhn V, Jämsä T. Association of geometric factors and failure load level with the distribution of cervical vs. trochanteric hip fractures. Journal of Bone and Mineral Research. 2006;21(6):895–901. doi: 10.1359/jbmr.060305. [DOI] [PubMed] [Google Scholar]
- 40.Szulc P, Seeman E, Duboeuf F, Sornay-Rendu E, Delmas PD. Bone fragility: failure of periosteal apposition to compensate for increased endocortical resorption in postmenopausal women. Journal of bone and mineral research. 2006;21(12):1856–1863. doi: 10.1359/jbmr.060904. [DOI] [PubMed] [Google Scholar]
- 41.Szulc P, Delmas P. Bone loss in elderly men: increased endosteal bone loss and stable periosteal apposition. The prospective MINOS study. Osteoporosis Int. 2007;18(4):495–503. doi: 10.1007/s00198-006-0254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cawthon PM, Ewing SK, McCulloch CE, Ensrud KE, Cauley JA, Cummings SR, et al. Loss of hip BMD in older men: the osteoporotic fractures in men (MrOS) study. Journal of Bone and Mineral Research. 2009;24(10):1728–1735. doi: 10.1359/JBMR.090419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cappola A, Hawkes W, Blocher N, Yu-Yahiro J, Orwig D, Fredman L, et al. The hormonal profile of hip fracture female patients differs from community-dwelling peers over a 1-year follow-up period. Osteoporosis Int. 2011;22(1):339–344. doi: 10.1007/s00198-010-1187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaptoge S, Dalzell N, Folkerd E, Doody D, Khaw K, Beck T, et al. Sex hormone status may modulate rate of expansion of proximal femur diameter in older women alongside other skeletal regulators. The Journal of Clinical Endocrinology & Metabolism. 2007;92(1):304–313. doi: 10.1210/jc.2006-0893. [DOI] [PubMed] [Google Scholar]
- 45.Travison TG, Araujo AB, Beck TJ, Williams RE, Clark RV, Leder BZ, et al. Relation between serum testosterone, serum estradiol, sex hormone-binding globulin, and geometrical measures of adult male proximal femur strength. The Journal of Clinical Endocrinology & Metabolism. 2009;94(3):853–860. doi: 10.1210/jc.2008-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lambert JK, Zaidi M, Mechanick JI. Male osteoporosis: epidemiology and the pathogenesis of aging bones. Current osteoporosis reports. 2011;9(4):229–236. doi: 10.1007/s11914-011-0066-z. [DOI] [PubMed] [Google Scholar]
- 47.Bor A, Matuz M, Gyimesi N, Biczók Z, Soós G, Doró P. Gender inequalities in the treatment of osteoporosis. Maturitas. 2015;80(2):162–169. doi: 10.1016/j.maturitas.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Antonelli M, Einstadter D, Magrey M. Screening and treatment of osteoporosis after hip fracture: comparison of sex and race. Journal of Clinical Densitometry. 2014;17(4):479–483. doi: 10.1016/j.jocd.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 49.Beck TJ, Oreskovic TL, Stone KL, Ruff CB, Ensrud K, Nevitt MC, et al. Structural adaptation to changing skeletal load in the progression toward hip fragility: the study of osteoporotic fractures. Journal of Bone and Mineral Research. 2001;16(6):1108–1119. doi: 10.1359/jbmr.2001.16.6.1108. [DOI] [PubMed] [Google Scholar]
- 50.Petit MA, Beck TJ, Lin H, Bentley C, Legro RS, Lloyd T. Femoral bone structural geometry adapts to mechanical loading and is influenced by sex steroids: the Penn State Young Women’s Health Study. Bone. 2004;35(3):750–759. doi: 10.1016/j.bone.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 51.Kersh ME, Pandy MG, Bui QM, Jones AC, Arns CH, Knackstedt MA, et al. The heterogeneity in femoral neck structure and strength. Journal of Bone and Mineral Research. 2013;28(5):1022–1028. doi: 10.1002/jbmr.1827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


