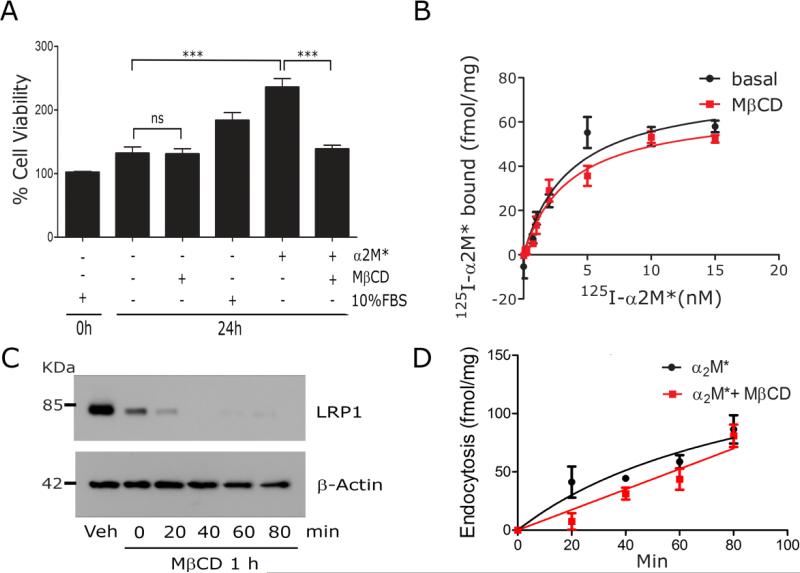
Fig. 4.
The ligand-binding and endocytic activity of LRP1 are unchanged by lipid raft disruption. (A) PC12 cells were cultured in SFM with 1 mM MβCD, 10 nM α2M*, or 10% FBS, as indicated. WST-1 cell viability assays were performed (mean ± SEM, ns, not statistically significant, ***p<0.001). (B) PC12 cells were treated with 1 mM MβCD or vehicle (basal) for 30 min and then equilibrated at 4° C. Specific binding of 125I-α2M* was determined. The results of three separate experiments were averaged. (C) PC12 cells were surface-labeled with biotin and then treated with 1 mM MβCD or vehicle for 30 min. The cultures were washed and the medium replaced with EHB medium (no MβCD) for the indicated times. Detergent-soluble “S” and -insoluble “I” fractions were prepared. Biotinylated proteins in the fractions were affinity-precipitated and LRP1 was detected by immunoblot analysis. Actin in unfractionated extracts was measured as a control for load. (D) PC12 cells were pre-treated with MβCD or vehicle. The cultures were washed and re-established in EHB with 5 nM 125I-α2M*, in the presence and absence of unlabeled α2M*, at 37° C. Internalized radioligand was determined as a function of time. Presented results represent three separate experiments.