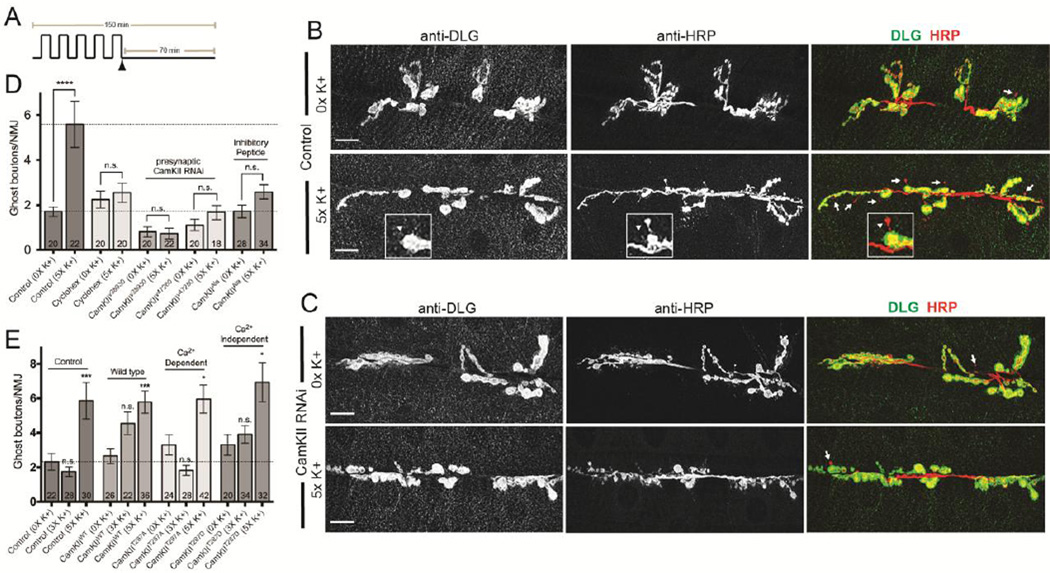
Figure 1. Presynaptic CamKII is required to control activity-dependent axon terminal growth.
(A) Diagram of the 5X high K+ spaced training paradigm. Partially dissected larval NMJ preps were exposed to a series of five pulses of HL3 containing high K+ (90 mM KCl) separated by rest periods in normal buffer (5 mM KCl). In experiments indicated, cyclohexamide (100 mM) was added to normal HL3 immediately preceding the final ~70 minute rest period (indicated by the arrowhead). (B and C) Larvae were exposed to 0X and 5X high K+ spaced synaptic stimulation and, at the end of the final rest period, preps were fixed and labeled with antibodies against postsynaptic DLG (green) and presynaptic HRP (red). Larger confocal images show an entire synaptic arbor at muscle 6 and 7 in abdominal segment 3. Arrows in (B) and (C) and the arrowhead in (B; inset) point to ghost boutons which are characterized as HRP+ and DLG- presynaptic extensions. Genotypes shown are (B) a Canton-S control and (C) a larval prep expressing a transgenic CamKII RNAi construct in motor neurons (C380 > CamKIIv38930). (D) The number of ghost boutons following 5X high K+ depolarization in Canton-S controls, Canton-S controls treated with cyclohexamide, and in different genetic backgrounds to either knock down presynaptic CamKII expression (C380>CamKIIv38930 and C380>CamKIIv47280) or disrupt its presynaptic function (C380>CamKIIAla). (E) The number of ghost boutons following 3X and 5X high K+ depolarization in controls (C380/+) and genetic backgrounds that express wild-type (C380>CamKIIWT) or mutant forms (C380>CamKIIT287A or C380>CamKIIT287D) of CamKII in motor neurons. (D and E) The numbers of NMJs analyzed are indicated within each column. * p < 0.05, *** p < 0.001, **** p < 0.0001. The error bars indicate the SEM.