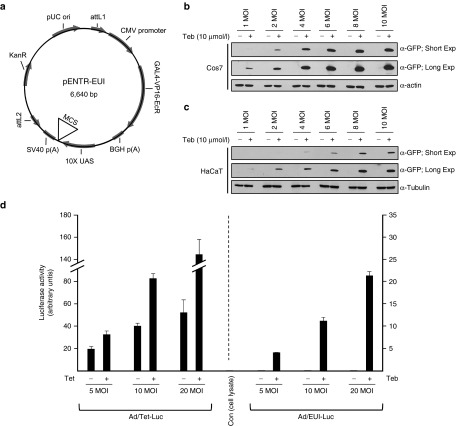
Figure 4.
An adenoviral singular gene switch induces tightly regulated transgene expression in cultured cell lines. (a) A schematic diagram of the pENTR-EUI vector optimized for transgene expression under the control of tebufenozide treatment. The Gal4-VP16-EcR sequence information is identical to that in the pEUI(+) vector. The nucleotide sequence of the multiple cloning site (MCS) of pENTR-EUI is identical to that of pEUI(+).Cos7 cells (b) or HaCaT cells (c) were treated with purified adenoviruses encoding the EGFP reporter gene for 24 hours at a multiplicity of infection (MOI) of 1–10. After exchanging the culture medium, cells were grown for 24 hours with tebufenozide (10 μmol/l) before immunoblotting with an anti-GFP antibody. The endogenous level of α-actin (b) or α-tubulin (c) was measured by western blotting to validate the equal loading of each sample. Short and long exposures show the range of responsiveness of the adenoviral vector to tebufenozide. (d) Comparison of the capacities of the pENTR-EUI and pENTR-Tet (Supplementary Figure S2)-based adenoviral vectors through the measurement of firefly Luc activity. HaCaT cells were treated with the indicated adenoviral particles at an increasing MOI. Note that cells treated with Ad/EUI-Luc did not show any detectable background expression in any experimental conditions (2.18-, 0.83-, and 1.36-fold increase with a low, medium, and high dose of virus particles, respectively, versus control cell lysate). Leaky transgene expression was increased in Ad/Tet-Luc-infected cells in a virus dosage-dependent manner (1,661-, 3,399-, and 4,431-fold increase with a low, medium, and high dose of virus particles, respectively, versus control cell lysate). Teb, tebufenozide; Tet, tetracycline.