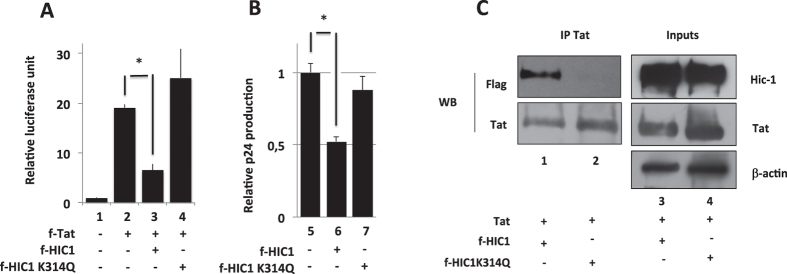
Figure 7. Acetylation of lysine 314 is detrimental to HIC1 inhibitory activity and disrupts HIC1/TAT association.
HIC1 central domain contains a MK314HEP conserved sequence, in which the lysine 314 (K314) acts as an acetylation/SUMOylation switch (Fig. 1). SIRT1 interacts with the HIC1 5 krueppel-like zinc fingers and deacetylates K314. SIRT1 recruits also HDAC4 which facilitate SUMOylation of K314 (Fig. 1). Microglial cells were co-transfected with the indicated constructs and either episomal pLTR-Luc (A) or pNL-4.3 viral genome (B). 48-hours post-transfection, cells were lysed and subjected to luciferase assay, while normalized with renilla luciferase system (A) or supernatants were harvested and p24 concentration titrated (B). Values are normalized relatively to basal level corresponding to empty vector (A,B), lane1. HEK293T cells were transfected with pFlag-HIC1 wild-type (C), lane 3 or the pFlag-HIC1 mutant K314Q (C), lane 4 in presence of TAT. Nuclear cell extracts were obtained after 48-hours transfection of the indicated vectors and subjected to western-blot experiments with anti-flag and anti-TAT antibodies (C), Inputs lanes 3 and 4. Cells were lysed and nuclear extracts have been subjected to immunoprecipitation with an anti-TAT antibody (C). Co-immunoprecipitations of Flagged HIC1 wild type and K314Q mutant with TAT were compared by western-blot with respectively anti-flag and anti-TAT antibodies (C) IP Tat lanes 1 and 2).