Abstract
Background:
Supratherapeutic international normalized ratio (INR) in patients on warfarin is a common side effect. Updated guidelines recommend against using vitamin K to correct INRs 4.5 to 10 in the absence of bleeding. The impact of compliance with updated guidelines during hospitalization has not been fully explored.
Methods:
A retrospective, observational study was performed utilizing electronic medical records. The goal was to evaluate management of supratherapeutic INR values for medicine inpatients and identify differences in clinical outcomes among inpatients treated and not treated with vitamin K. Records from adult inpatients with at least one INR value between 4.5 and 9 were reviewed. A total of 51 records were evaluated. Thirty-four patients did not receive vitamin K compared to 17 who did. Bleeding events, readmissions rates, length of stay, and familiarity with new guidelines were studied.
Results:
Mean age of patients was 73 years, and 71% were female. No statistically significant differences were observed in bleeding events between patients who received vitamin K and those who did not: 2/17 (12%) and 1/34 (3%), respectively (P = .30). No differences in 30-day readmission rates (24% vs 18%; P = .71) or in length of stay (7 vs 4 days; P = .11) were found. All pharmacists (13 of 13) were familiar with CHEST 2012 guidelines on the management of supratherapeutic INR compared to 10 of 21 (48%) hospitalists (P = .001).
Conclusions:
With the national focus on reduction of health care costs, health systems are looking at innovative ways to reduce readmission rates and length of stay. This study, which evaluated the use of vitamin K administration, showed no statistical difference between bleeding events, readmission rates, and length of stay in patients who received vitamin K. Education on the updates of guidelines may be beneficial, as many providers were not familiar with the changes in recommendations.
Keywords: anticoagulation, hospitalization, international normalized ratio, vitamin K, warfarin
Nationally, anticoagulants contribute to approximately 10% of all drug-related inpatient adverse events.1 Warfarin has been one of the most widely used oral anticoagulants, but it is associated with increased bleeding risks.2 Although newer target-specific oral anticoagulants are also associated with bleeding, they currently do not require monitoring international normalized ratio (INR) values as the use of warfarin requires. Supratherapeutic INR in patients on chronic warfarin therapy is a common side effect.3,4 Holbrook et al3 noted that the yearly incidence of warfarin-associated bleeding was approximately 1% to 3%. Moreover, individuals on warfarin are 5 times more likely to bleed than those not on warfarin.5 Phytonadione (vitamin K) is used as a reversal agent for excessive anticoagulation with warfarin; it is known to reduce INRs faster than placebo.6 A randomly controlled trial to assess management of supratherapeutic INR values 4.5 to 10 in nonbleeding patients found similar bleeding rates in patients who received vitamin K as in those who did not.7
Updated 2012 CHEST guidelines (9th ed) recommend against the use of routine vitamin K administration while withholding warfarin for INR values 4.5 to 10 in patients without evidence of bleed.3 The 2008 CHEST guidelines (8th ed) recommended withholding warfarin and administering low-dose vitamin K for reversal of supratherapeutic INR.8 There are several concerns with administering vitamin K, such as anaphylaxis, overcorrection of therapeutic target INR range, and warfarin resistance once warfarin is restarted. Although rare, anaphylaxis has been associated with the intravenous dosage form of vitamin K (estimated incidence rate of 3 cases per 10,000 doses).9 Overcorrection of therapeutic INR and warfarin resistance can render patients unprotected from thrombosis. The clinical implications of implementation of the new guidelines, such as hospital cost, inpatient outcomes, and provider knowledge, have not been completely investigated.
In this retrospective comparison study, we hypothesized that compliance with the 2012 CHEST guidelines would lead to reduced utilization of hospital resources and improvement in patient outcomes. The primary outcome measure was bleeding events noted in the setting of administering or withholding vitamin K in nonbleeding patients with an INR 4.5 to 9. Secondary measures included length of stay, readmissions, and provider awareness of updated CHEST guidelines for the use of vitamin K.
Methods
This single-center study was conducted at a city-based, 545-bed academic teaching hospital. Medicine services comprised of both house staff and hospitalists and clinical pharmacy services are available on all units. Eligibility criteria for selected patients were at least one INR value 4.5 to 9 in adult patients admitted to the general medical service (9 was chosen as the upper limit of the INR range because laboratory restrictions at our institution do not report values >9.1). Exclusion criteria included INR values greater than or equal to 9.1, patients with evidence of active bleeding within first 24 hours of admission, use of vitamin K for indications other than warfarin reversal, emergent surgeries, or any intensive and intermediate care unit transfers. Electronic medical records for eligible patients hospitalized from December 2012 to May 2013 were reviewed.
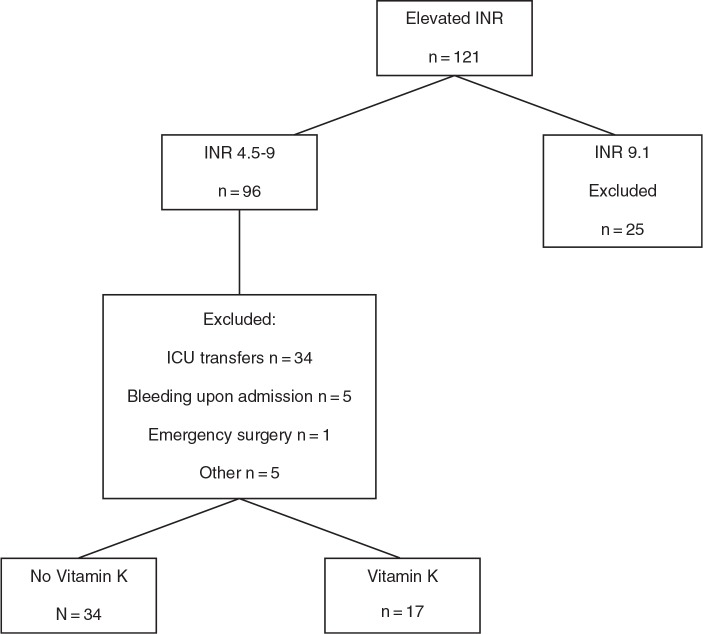
During this selected time frame, there were 121 patients with an initial INR greater than 4.5 (Figure 1). Forty-five patients with INRs ranging from 4.5 to 9 met exclusion criteria. Twenty-five patients with initial INRs greater than 9.1 were also excluded. Therefore, the study compared 51 patients. Seventeen patients received vitamin K and 34 did not.
Figure 1.
Patient selection. ICU = intensive care unit; INR = international normalized ratio.
Providers and pharmacists were surveyed to assess their familiarity with the 2012 CHEST guidelines, awareness of recommendation changes from the 2008 guidelines, and preferred resources for the management of elevated INR values. The survey was sent to all hospitalists and hospital pharmacists. Thirty-four of 63 health care professionals responded to the online survey.
The following is an excerpt from the survey sent to health care professionals to evaluate their familiarity with CHEST guidelines and to evaluate practice trends of management of supratherapeutic INR values secondary to warfarin:
-
1.
Are you familiar with the American College of Chest Physicians (CHEST) Antithrombotic Therapy and Prevention of Thrombosis 2012 guidelines, specifically the section pertaining to supratherapeutic INR management? Yes/No
-
2.
Did you know CHEST changed its position from its 2008 to 2012 edition guidelines regarding the use of vitamin K for INR values 4.5–9? Yes/No
-
3.
If yes for question 2, did it change the way you manage supratherapeutic INR values? Yes/No. Please explain.
Patient information was collected through the hospital’s profile data system (Meditech). Bleeding events, readmissions rates, length of stay, and survey results were analyzed by Fisher exact test, Mann-Whitney U test, and chi-square test, respectively.
The primary endpoint was the incidence of any documented bleeding event after 24 hours of admission in patients who received vitamin K compared to those who did not. Bleeding was noted in the provider’s written notes (where bleeding could be described as but not limited to development of new hematomas, epistaxis, hematemesis, hematochezia, and melena). Secondary endpoints were length of hospital stay and readmission rates. Differences in the familiarity of health care professionals with CHEST 2012 guidelines, their awareness of recommended changes, and their primary resource for management were examined by the survey. This study was conducted in accordance with the institutional review board (IRB)–approved protocol.
Results
Baseline patient characteristics were similar between the 2 groups (Table 1), although the group receiving vitamin K was more often female (59% vs 35%; P = .234). Risk factors for bleeding included past use of aspirin (acetylsalicylic acid [ASA]) or nonsteroidal anti-inflammatory drugs (NSAIDs), history of stroke, history of gastrointestinal bleeding, malignancy, hepatic insufficiency, renal insufficiency, and warfarin sensitivity (Table 2). There was no statistical difference regarding risk factors for bleeding between the 2 groups. Twenty-two doses of vitamin K were administered in 17 patients; 29% of whom received more than 1 dose. The most common dose given was 2.5 mg (2.5–5 mg), and the most common route was oral followed by subcutaneous (91% and 9%, respectively). The average INR for which vitamin K was administered was 6.6 (SD 1.7). At discharge, 38% of patients who did not receive vitamin K left the hospital with a supratherapeutic INR (38% vs 23%; P = .372).
Table 1. Baseline patient characteristics.
| Characteristics | No vitamin K (n = 34) | Vitamin K (n = 17) |
|---|---|---|
| Age, years, mean ± SD | 70± 15 | 76± 12 |
| Male, n (%) | 22 (65) | 7 (41) |
| Indication for anticoagulation, n (%) | ||
| Atrial fibrillation | 23 (68) | 9 (53) |
| Venous thromboembolism | 10 (29) | 5 (29) |
| Mitral valve replacement | 0 (0) | 2 (12) |
| Other | 1 (3) | 1 (6) |
| Liver function tests, IU/L | ||
| AST, median (IQR) | 29 (14–39) | 26 (19–32) |
| ALT, median (IQR) | 25 (18–34) | 23 (18–41) |
| Serum creatinine, mg/dL, median (IQR) | 1.3 (0.98–1.75) | 1.2 (0.85–1.95) |
Note: ALT = alanine aminotransferase; AST = aspartate aminotransferase; IQR = interquartile range. P > .05 using chi-square test for categorical variables and Mann-Whitney U test for continuous data.
Table 2. Risk factors for bleeding in patients with elevated international normalized ratio: No vitamin K therapy received versus vitamin K received.
| Risk factors | No vitamin K (n = 34) | Vitamin K (n = 17) |
|---|---|---|
| Past medical history, n (%) | ||
| Renal insufficiency | 12 (35) | 10 (59) |
| ASA/NSAID use | 7 (21) | 5 (29) |
| History of stroke/TIA | 5 (15) | 0 (0) |
| Hepatic insufficiency | 4 (12) | 1 (6) |
| Malignancy | 4 (12) | 6 (35) |
| History of GI bleed | 1 (3) | 2 (12) |
| Warfarin weekly dose, mg, mean± SD | 38± 19 | 24± 8 |
Note: ASA = acetylsalicylic acid; GI = gastrointestinal; NSAID = nonsteroidal anti-inflammatory drug; TIA = transient ischemic attack. P > .05 using chi-square test for categorical variables and Mann-Whitney U test for continuous data.
There was no statistical difference in occurrence of bleeding events between patients who received vitamin K and those who did not (2/17 [12%] vs 1/34 [3%]; P = .255). There were a total of 3 bleeding events: gastrointestinal bleed, subconjunctival hemorrhage, and hemorrhagic pleural effusions in the setting of malignancy. Patients who received vitamin K had more readmissions within 30 days of discharge (4/17 [24%] vs 6/34 [18%]; P = .714) and had longer lengths of stay (7 days vs 4 days; P = .112) than patients who did not receive vitamin K, although this was not statistically significant. The etiologies for readmissions included congestive heart failure exacerbation, subsequent supratherapeutic INR values, cellulitis, pneumonia, syncope, and gastrointestinal upset. There was no evidence of the development of thromboembolism diagnosed during the hospital visit or subsequent readmissions.
Based on survey results, there was a statistically significant difference between the familiarity of prescribers and pharmacists with changes in recommended guidelines. All pharmacists (100%) were familiar with guidelines pertaining to management of supratherapeutic INR values compared to 48% of providers (P = .0018). Ninety-two percent of hospital pharmacists were aware of the recommendation changes from the 2008 to 2012 guidelines compared to 29% of providers. The survey showed that 29% of providers utilized guidelines for management of elevated INR compared to 70% of pharmacists (Table 3).
Table 3. Survey of health care professionals regarding practice trends in the management of supratherapeutic international normalized ratio (INR) values secondary to warfarin.
| Providers (physicians and physician assistants) (n = 21) | Hospital pharmacists (n = 13) | P | |
| Familiarity with 2012 CHEST guidelines, n (%) | 10 (48) | 13 (100) | .0018 |
| Awareness of change in vitamin K administration guidelines from 2008 to 2012 CHEST, n (%) | 6 (29) | 12 (92) | .0004 |
| Resource preference for management of elevated INR values, n (%) | |||
| Tertiary source | 19 (90) | 8 (62) | .0747 |
| Guidelines | 6 (29) | 9 (70) | .1945 |
| Colleagues | 2 (10) | 3 (23) | .7334 |
| Other | 1 (5) | 1 (7) | .8349 |
Discussion
Our study found that administering vitamin K for supratherapeutic INR does not result in a statistically significant reduction in bleeding events during hospital admission. These results are comparable to findings appreciated in previous studies.7 This suggests that withholding vitamin K is safe in nonbleeding patients with INR values 4.5 to 9. We found a trend toward increased health care resource utilization with the administration of vitamin K for elevated INR values. There was a trend toward a longer length of hospital stay and higher readmission rates in patients who received vitamin K. The reasons for this trend is not clear, but it is possible that vitamin K administration led to subtherapeutic INR values, which in turn required bridging upon reinitiating anticoagulation therapy. Furthermore, patients discharged with subtherapeutic INR values in the setting of comorbidities may be at risk for complications and readmissions.
A recent study found that hospitalized patients with atrial fibrillation benefit from vitamin K antagonist therapy by having a lower mortality. This may suggest that the use of vitamin K therapy in hospitalized patients may be harmful when not indicated. A major limitation of that study was that INR values were not assessed.10
Literature has reported that educational interventions improve compliance for appropriate use of vitamin K therapy.11 Additionally, pharmacist guidance with warfarin administration has shown greater achievement of therapeutic INR.12–15 Combined with findings from our survey, this suggests that pharmacists can play an important role in ensuring proper management of vitamin K therapy.
This study had some notable limitations. Retrospective studies are subject to selection biases and confounding variables. Other limitations of the study include its single-center design and small sample size. Given the small sample size, we were unable to carry out a power analysis. Due to the exclusion criteria of intensive care unit patients, sicker patients with major bleeding may have been excluded, leading to a reduced number of reported bleeding events. Assessment of bleeding events depended on provider documentation, which could be incomplete or subjective. Also, the upper limit of INR values at our institution was 9, and thus individuals with INRs between 9 and 10 were not captured in the study. Additionally, an examination of patients’ CHA2DS2-VASc scores was not carried out, as this also relies on complete and accurate provider documentation.
Based on our literature review, this is the first study that examined the impact that vitamin K administration may have on health care resources. It does not appear as if there is a significant impact on length of stay or readmission rates, which may lead to higher health care cost. Larger, multicenter studies conducted over a longer time period may determine whether the 2012 CHEST guidelines in regard to vitamin K use contribute to lower health care utilization.
Conclusions
With the national focus on the reduction of health care costs, health systems are looking at innovative ways to reduce readmission rates and length of stay. This study, which evaluated the use of vitamin K administration, showed no statistical difference between bleeding events, readmission rates, and length of stay in patients who received vitamin K. Education on the updates of guidelines may be beneficial, as many providers were not familiar with the changes in recommendations. Future studies are warranted to evaluate the effect of vitamin K administration on health care resources.
Acknowledgments
The authors have no financial disclosures to declare and no conflicts of interest to report.
The study was approved by the Johns Hopkins School of Medicine’s Institutional Review Board.
References
- 1.Lucado J, Paez K, Elixhauser A. Medication-related adverse outcomes in U.S. hospitals and emergency departments 2008; Statistical Brief #109. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. 2011. US Department of Health and Human Services, Agency for Healthcare Research and Quality. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb109.pdf. [PubMed]
- 2.Pirmohamed M. Warfarin: Almost 60 years old and still causing problems. Br J Clin Pharmacol. 2006;62(5):509–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holbrook A, Schulam S, Witt DM, et al. Evidence-based management of anticoagulation therapy: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. CHEST. 2012;141(suppl 2):e152S–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use: A prevalent adverse effect resulting in regulatory action. Arch Intern Med. 2007;167(13):1414–1419. [DOI] [PubMed] [Google Scholar]
- 5.Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: Clinical epidemiology, prediction, and prevention. Am J Med. 1993;95(3):315–328. [DOI] [PubMed] [Google Scholar]
- 6.Crowther MA, Ageno W, Garcia D, et al. Oral vitamin K versus placebo to correct excessive anticoagulation in patients receiving warfarin: A randomized trial. Ann Intern Med. 2009;1505:293–300. [DOI] [PubMed] [Google Scholar]
- 7.Crowther MA, Julian J, McCarty D, et al. Treatment of warfarin-associated coagulopathy with oral vitamin K: A randomised controlled trial. Lancet. 2000;3569241:1551–1553. [DOI] [PubMed] [Google Scholar]
- 8.Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines, 8th ed. CHEST. 2008;133:160S. [DOI] [PubMed] [Google Scholar]
- 9.Riegert-Johnson DL, Volchck GW. The incidence of anaphylaxis following intravenous phytonadione (vitamin K1): A 5-year retrospective review. Ann Allergy Asthma Immunol. 2002; 89(4):400–406. [DOI] [PubMed] [Google Scholar]
- 10.Lip GY, Clementy N, Pericart L, Banerjee A, Fauchier L. Stroke and major bleeding risk in elderly patients aged ≥75 years with atrial fibrillation: The Loire Valley Atrial Fibrillation Project [published online ahead of print November 25, 2014]. Stroke. [DOI] [PubMed]
- 11.Van Berkel MA, Crannage AJ, Murphy JA. Evaluation of education on the appropriate use of vitamin K in warfarin reversal in adult inpatients. Hosp Pharm. 2013;48(8):662–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chilipko AA, Norwood DK. Evaluating warfarin management by pharmacists in a community teaching hospital. Consult Pharm. 2014;29(2):95–103. [DOI] [PubMed] [Google Scholar]
- 13.Gupta V, Kogut SJ, Thompson S. Evaluation of differences in percentage of international normalized ratios in range between pharmacist-led and physician-led anticoagulation management services [published online ahead of print December 30, 2013]. J Pharm Pract. [DOI] [PubMed]
- 14.Young S, Bishop L, Twells L, Dillon C, Hawboldt J, O’Shea P. Comparison of pharmacist managed anticoagulation with usual medical care in a family medicine clinic. BMC Fam Pract. 2011;12:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong YM, Quek YN, Tay JC, Chadachan V, Lee HK. Efficacy and safety of a pharmacist-managed inpatient anticoagulation service for warfarin initiation and titration. J Clin Pharm Ther. 2011;36(5):585–591. [DOI] [PubMed] [Google Scholar]