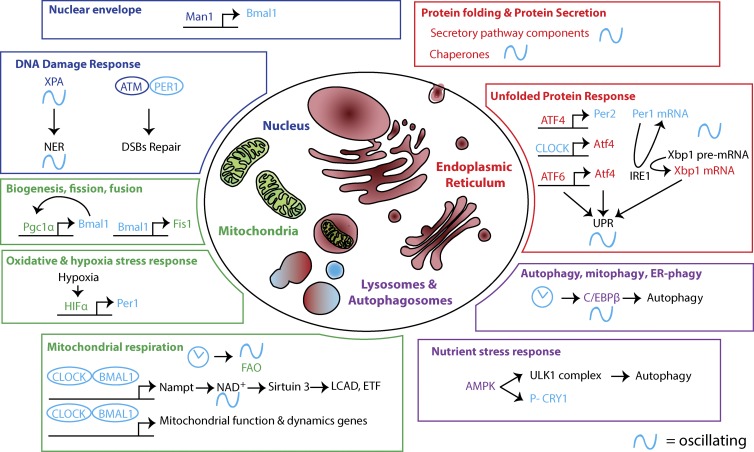
Figure 3.
Circadian oscillations in intracellular organelle’s structure and function. Intracellular organelles play critical cellular functions. Recently, their structure and function have been shown to be time-of-day dependent. In the nucleus (blue), the NE protein Man1 binds to Bmal1 promoter, and disruption of NE proteins affects CLOCK oscillations. DNA damage responses (NER and double-strand breaks [DSBs]) also show circadian rhythms because of the circadian oscillations in the level of xeroderma pigmentosum A (XPA) and the interaction of ataxia telangiectasia mutated (ATM) with PER1. In the mitochondria (green), mitochondria biogenesis is circadian owing to the reciprocal interaction between Pgc1α and Bmal1. Furthermore, Bmal1 controls the expression of Fis1, which plays a major role in mitochondrial dynamics (fission and fusion). Oxidative and hypoxia stress responses have a circadian component. Reciprocally, hypoxia affects the clock via hypoxia-inducible factor 1α (HIF1α)–mediated transcriptional control of Per1. The circadian clock is required for oscillation of mitochondrial metabolic activity, in particular FAO. CLOCK/BMAL1 entrains cyclical mitochondrial function and gene expression. Furthermore, they control the expression of nicotinamide phosphoribosyltransferase (Nampt), which drives a circadian rhythm in the level of NAD+. Sirt3 activity depends on the level of the cofactor NAD+. Because they are targets of Sirt3, the activity of the FAO enzymes long-chain acyl coenzyme A dehydrogenase (LCAD) and electron-transferring flavoprotein (ETF) oscillates. Additional mechanisms likely contribute as well. In the lysosomes (purple), upon nutrient deprivation, the kinase AMPK activates the ULK1 complex, which induces autophagy to recycle cellular energy. AMPK also controls the circadian clock by phosphorylating CRY1. The clock also drives rhythmic expression of CCAAT enhancer–binding protein β (C/EBPβ), a master regulator of transcriptional activation of autophagy. In the ER (red), the expression of components of the secretory pathway is circadian. In hepatocytes, this happens in sync with feeding, when liver exocrine and endocrine functions are at a maximum. The activity of ER-resident PRX, which is essential for protein folding and maintaining the redox state in the ER, also is circadian. The strength of UPR activation in the ER is time-of-day dependent. Clock-mediated transcription of ATF4 and the circadian oscillation in Xbp1 pre-mRNA splicing are contributing mechanisms. Reciprocally, the UPR modulates the clock via feedback from IRE1-mediated splicing of Per1 mRNA and ATF4-driven transcription of Per2.