Abstract
We present a proposition, the "poly(L-alanine) hypothesis," which asserts that the native backbone geometry for any polypeptide or protein of M residues has a closely mimicking, mechanically stable, image in poly(L-alanine) of the same number of residues. Using a molecular mechanics force field to represent the relevant potential energy hypersurfaces, we have carried out calculations over a wide range of M values to show that poly(L-alanine) possesses the structural versatility necessary to satisfy the proposition. These include poly(L-alanine) representatives of minima corresponding to secondary and supersecondary structures, as well as poly(L-alanine) images for tertiary structures of the naturally occurring proteins bovine pancreatic trypsin inhibitor, crambin, ribonuclease A, and superoxide dismutase. The successful validation of the hypothesis presented in this paper indicates that poly(L-alanine) will serve as a good reference material in thermodynamic perturbation theory and calculations aimed at evaluating relative free energies for competing candidate tertiary structures in real polypeptides and proteins.
Full text
PDF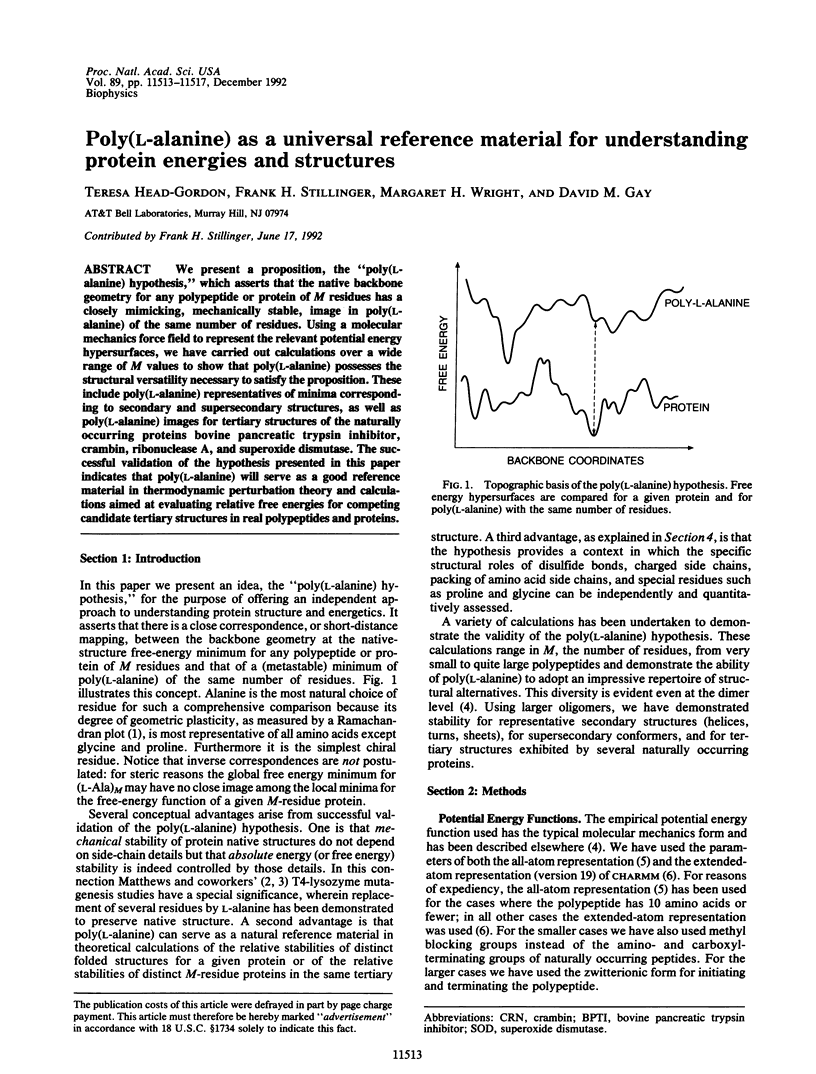




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eriksson A. E., Baase W. A., Zhang X. J., Heinz D. W., Blaber M., Baldwin E. P., Matthews B. W. Response of a protein structure to cavity-creating mutations and its relation to the hydrophobic effect. Science. 1992 Jan 10;255(5041):178–183. doi: 10.1126/science.1553543. [DOI] [PubMed] [Google Scholar]
- Hagler A. T., Honig B. On the formation of protein tertiary structure on a computer. Proc Natl Acad Sci U S A. 1978 Feb;75(2):554–558. doi: 10.1073/pnas.75.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head-Gordon T., Stillinger F. H., Arrecis J. A strategy for finding classes of minima on a hypersurface: implications for approaches to the protein folding problem. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11076–11080. doi: 10.1073/pnas.88.24.11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartha G., Bello J., Harker D. Tertiary structure of ribonuclease. Nature. 1967 Mar 4;213(5079):862–865. doi: 10.1038/213862a0. [DOI] [PubMed] [Google Scholar]
- Levitt M., Warshel A. Computer simulation of protein folding. Nature. 1975 Feb 27;253(5494):694–698. doi: 10.1038/253694a0. [DOI] [PubMed] [Google Scholar]
- RAMACHANDRAN G. N., RAMAKRISHNAN C., SASISEKHARAN V. Stereochemistry of polypeptide chain configurations. J Mol Biol. 1963 Jul;7:95–99. doi: 10.1016/s0022-2836(63)80023-6. [DOI] [PubMed] [Google Scholar]
- Rees D. C., Lewis M., Lipscomb W. N. Refined crystal structure of carboxypeptidase A at 1.54 A resolution. J Mol Biol. 1983 Aug 5;168(2):367–387. doi: 10.1016/s0022-2836(83)80024-2. [DOI] [PubMed] [Google Scholar]
- Tainer J. A., Getzoff E. D., Beem K. M., Richardson J. S., Richardson D. C. Determination and analysis of the 2 A-structure of copper, zinc superoxide dismutase. J Mol Biol. 1982 Sep 15;160(2):181–217. doi: 10.1016/0022-2836(82)90174-7. [DOI] [PubMed] [Google Scholar]
- Tobias D. J., Sneddon S. F., Brooks C. L., 3rd Reverse turns in blocked dipeptides are intrinsically unstable in water. J Mol Biol. 1990 Dec 5;216(3):783–796. doi: 10.1016/0022-2836(90)90399-7. [DOI] [PubMed] [Google Scholar]
- Zhang X. J., Baase W. A., Matthews B. W. Toward a simplification of the protein folding problem: a stabilizing polyalanine alpha-helix engineered in T4 lysozyme. Biochemistry. 1991 Feb 26;30(8):2012–2017. doi: 10.1021/bi00222a001. [DOI] [PubMed] [Google Scholar]






