Figure 1. HP1α interacts with PC2 to regulate PC's SUMO E3 ligase activity.
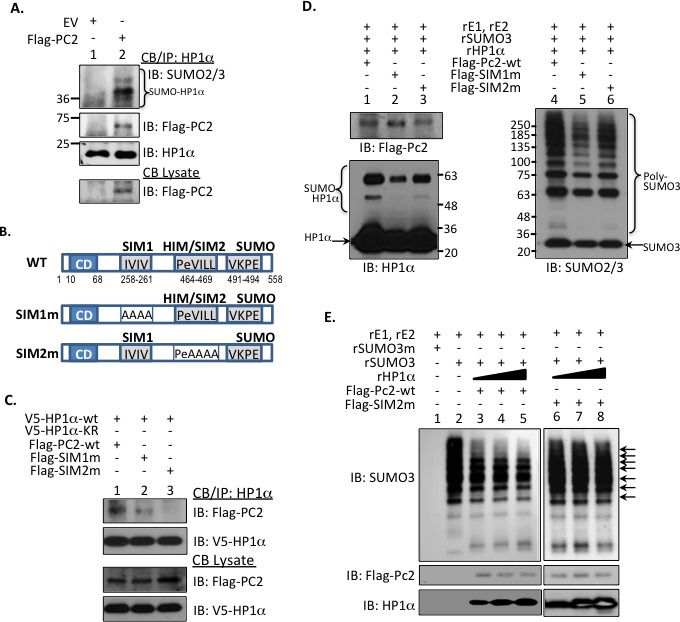
A. Chromatin-bound endogenous HP1α associates with and is a target for PC2. Endogenous HP1α was immunoprecipitated from MCF7 cells expressing empty vector (EV) or flag-tagged PC2. Immunoblots using SUMO2/3 and Flag antibodies confirmed HP1α modification and protein-protein interaction. B. Schematic representation of PC2's 2 SUMO-interaction motifs (SIM), HP1α-interaction motif (HIM), and single SUMO-conjugation site in relation to the chromodomain (CD). The hydrophobic amino acids were mutated to alanine to generate the appropriate SIM1 and SIM2 mutants. C. HP1α-interaction motif is required for PC2-HP1α binding. MCF7 cells were incubated with either wild-type or PC2 plasmids with mutations to either 1 of the 2 SIMs. Association of PC2 with HP1α was assessed via SDS-PAGE. D. PC2-SIM1 affects in vitro HP1α SUMOylation. Purified Flag-tagged wild-type or SIM-mutant PC2 was incubated with recombinant SUMO machinery and HP1α and subsequently subject to immunoblotting. Poly-SUMO chains (brackets labeling SUMO-HP1α and Poly-SUMO3) and unmodified HP1α and SUMO3 protein (arrows) is indicated. E. The E3 activity of wt-PC2, but not SIM2m, is reduced with increasing concentrations of HP1α. An in vitro SUMOylation assay was used to evaluate poly-SUMO chains in the presence of increasing recombinant HP1α. Arrows SUMO3 bands in PC2-wt samples affected by the addition of recombinant HP1α (rHP1α).
