Figure 6. Proposed model for the role of EVs and CD30 shedding for the immunotargeting with SGN-35.
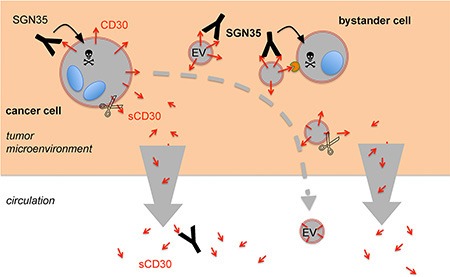
The malignant cells in cHL reside in lymphoid tissue, surrounded by a microenvironment of extracellular matrix and proinflammatory cells. They selectively express the receptor CD30. The CD30 antibody-drug conjugate SGN-35 binds to the CD30+ tumor cells, is internalized and the toxic compound is cleaved and activated by lysosomal proteases. The malignant cells not only expose CD30 on the surface, they also release CD30 on EVs (CD30EV), either by membrane blebbing or release of exosomes from multivesicular endosomes. EVs also bind SGN-35 and SGN-35-loaded EVs migrate away from the cancer cell. The loading of EVs might also occur within the tumor cell. After SGN-35 internalization, the drug might target to multivesicular endosomes instead of lysosomes. Apoptotic blebs of damaged tumor cells might contribute to the release of SGN-35-loaded EVs. Mast cells and eosinophils support the cHL tumor growth. These cells express the natural CD30 ligand and bind SGN-35-loaded CD30EV. These cells might internalize the EVs and are damaged by SGN-35 in a CD30EV-dependend manner. Cells and EVs express the CD30 sheddase ADAM10, which gradually cleaves the ectodomain. In the microenvironment, sCD30 is quickly drained whereas EVs are retained. Monomeric sCD30 is a competitor of SGN35 binding to cells and EVs. This hypothesis suggests an elevated ratio of membrane-associated CD30 within the matrix and an elevated ratio of sCD30 outside of the matrix. SGN-35 exploits this mechanism to preferentially target cancer and bystander cells in the tumor microenvironment. The CD30-depleted EVs and the high level of competing sCD30 in the circulation might explain the minute side-effects of the drug.
