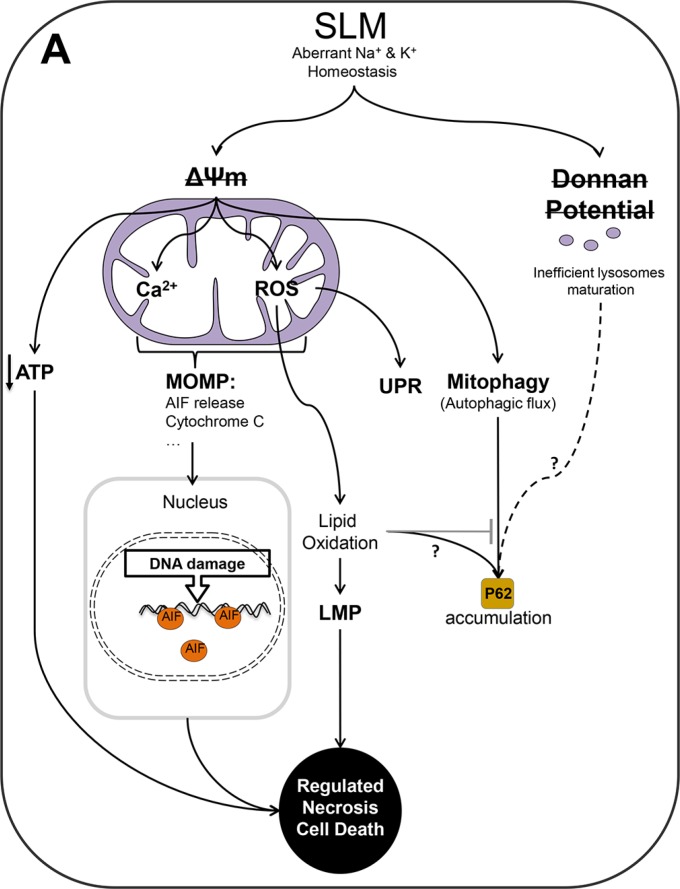
Figure 7. Schematic representation of the authors' proposed model of the action of SLM in glioblastoma cells.
(A) SLM induces a direct alteration in mitochondrial ΔΨ and as consequence a disruption in the Donnan potential of the lysosomal membrane. SLM thus partially inhibits lysosome maturation leading to a reduction in the amount of active cathepsin (B) Although there is no doubt that SLM induces an aberrant autophagic flux, the role of the Donnan potential in this respect is not clear. In the case of mitochondria, SLM induced a decrease in the ΔΨ and an increase in the levels of intra-mitochondrial calcium and ROS. In our proposed model, this oxidative stress triggers the UPR. The elevated levels of calcium and ROS inside mitochondria results in MOMP which leads to release of AIF. Once AIF spreads into the nucleus, it damages DNA through its endonuclease activity. The presence of AIF would establish a feedback loop that would cause cell death by a mechanism of regulated necrosis. In parallel to this, we propose that it should not be ruled out that the action of SLM involves other different molecular routes that could lead to regulated necrosis. For example, a mitochondrial membrane alteration would directly cause a deficit of ATP, which would cause energetic failure, which would, in turn, provoke cell death by regulated necrosis. In addition, impairment of mitochondrial activity would trigger mitophagy, with engulfment of the organelle and initiation of autophagy. A key role in our proposed model is played by oxidative stress, which blocks autophagic flux, probably through a process involving lipid oxidation.