Abstract
Background:
Our aim was to compare the accuracy of lung ultrasound (LUS) and standard chest x-ray (CXR) for diagnosing pneumonia in older patients with acute respiratory symptoms (dyspnea, cough, hemoptysis, and atypical chest pain) admitted to an acute-care geriatric ward.
Methods:
We enrolled 169 (80 M, 89 F) multimorbid patients aged 83.0 ± 9.2 years from January 1 to October 31, 2015. Each participant underwent CXR and bedside LUS within 6 hours from ward admission. LUS was performed by skilled clinicians, blinded to CXR results and clinical history. The final diagnosis (pneumonia vs no-pneumonia) was established by another clinician reviewing clinical and laboratory data independent of LUS results and possibly prescribing chest contrast-enhanced CT. Diagnostic parameters of CXR and LUS were compared with McNemar test on the whole cohort and after stratification for Rockwood Clinical Frailty Scale.
Results:
Diagnostic accuracy for pneumonia (96 patients) was significantly higher in LUS (0.90, 95% confidence interval [CI] 0.83–0.96) compared with CXR (0.67, 95%CI 0.60–0.74, P < 0.001). LUS had a better sensitivity (0.92, 95%CI 0.86–0.97 vs 0.47, 95%CI 0.37–0.57) and negative predictive value (0.95, 95% CI 0.83–0.96 vs 0.57, 95%CI 0.48–0.56). In those patients with frailty (n = 87 with Rockwood Clinical Frailty Scale ≥5), LUS maintained a high diagnostic accuracy, but CXR did not (P = 0.0003). Interobserver agreement for LUS, calculated in a subsample of 29 patients, was high (k = 0.90).
Conclusions:
In multimorbid patients admitted to an acute geriatric ward, LUS was more accurate than CXR for the diagnosis of pneumonia, particularly in those with frailty. A wider use of LUS should be implemented in this setting.
Keywords: bedside ultrasound, chest radiography, chest ultrasound, frailty, geriatric care, pneumonia
1. Introduction
In adult patients with acute respiratory symptoms, lung ultrasound (LUS) is a useful test for diagnosing pneumonia, especially when chest x-ray (CXR) results are negative or inconclusive.[1–3] Guidelines still consider CXR as the reference first-line diagnostic test in all patients with suspected pneumonia. However, its diagnostic accuracy is not optimal, due to high interobserver variability in interpretation.[4] Patient-related factors may also bias the acquisition of a good radiograph, especially in those with severe symptoms.[5] These limitations may increase the number of chest CT prescriptions.[6] Conversely, the routine application of LUS in emergency departments and intensive care units (ICUs) is associated with an improvement of diagnostic accuracy for pneumonia and may even in some cases replace CXR,[7,8] reducing the need of CT scans.[9]
As such, some authors recommend LUS as a part of standard diagnostic workup for management of patients with acute respiratory symptoms.[10] However, since about 8% of pneumonic lesions are not detectable by LUS for anatomic reasons,[11] other authors recommend its use as a complementary diagnostic test.[12]
To date, virtually no single clinical study investigating the diagnostic accuracy of LUS for pneumonia has been focused exclusively on older individuals who need hospital admission in internal medicine or geriatric wards for respiratory symptoms.[1] Pneumonia in this population is however a frequent concern, representing one of the most frequent diagnoses in patients over age 65.[13] Atypical symptoms, like confusion and unsteadiness, are also frequent in this setting, making the final diagnosis challenging.[14] Frailty syndrome, functional disability, cognitive impairment, and multimorbidity have a high prevalence in this age group.[15–17] These factors may predispose toward poor-quality radiographic images, due to chronic underlying diseases, lack of cooperation, and difficulty maintaining posture.
In many countries, the application of bedside ultrasonography in hospital practice represents a de facto standard for initial assessment of a wide range of clinical situations, especially in older individuals with mobility limitations.[18] However, it is still unclear whether ultrasound can improve diagnostic algorithms for pneumonia in this setting, especially in those cases in which mobility limitations and poor cognitive performance may bias the quality of CXR images.
Thus, the aim of the present study was to evaluate the diagnostic accuracy for pneumonia of bedside LUS and CXR (index tests), compared with comprehensive clinical and laboratory evaluation (reference standard), in a cohort of multimorbid frail elderly acutely hospitalized with respiratory symptoms, also stratifying for functional performance according to the Rockwood model.[19]
2. Methods
2.1. Study population
We prospectively enrolled all consecutive elderly (age ≥65) multimorbid (≥2 chronic diseases) patients with acute respiratory complaints urgently admitted from emergency department to a teaching geriatric hospital ward in Parma, Italy, from January 1 to October 31, 2015.
Subjects were included in the study if they had at least one of the following acute symptoms: dyspnea, cough, hemoptysis, and atypical chest pain (i.e., pleuritic pain). Unexplained fever without extrathoracic symptoms and localized absence of breath sounds or crackles on lung auscultation were adjunctive inclusion criteria. Patients with terminal disease (estimated survival <1 month), known chronic respiratory symptoms, and/or lung cancer were excluded from the study. Since the timing of CXR and LUS may influence results and their interpretation, LUS performance greater than 6 hours from CXR execution in emergency department was also considered as an exclusion criterion.
As highlighted in Fig. 1, the number of patients eligible for study inclusion in the considered time span was 270. From these, 101 were excluded (23 for terminal illness, 52 for established chronic causes of respiratory symptoms, and 26 for LUS performance greater than 6 hours from CXR scan). Thus, the final study population was composed of 169 subjects (80 M, 89 F).
Figure 1.
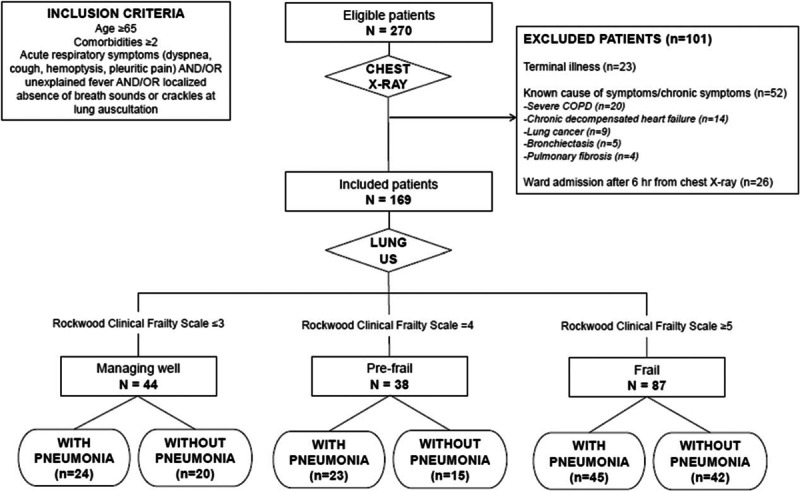
Summary of the study design, stratification of included patients according to the cumulative deficit frailty model (Rockwood Clinical Frailty Scale) and final diagnosis (presence/absence of pneumonia). COPD = Chronic Obstructive Pulmonary Disease.
2.2. Diagnostic examinations and study protocol
All participants underwent a standard CXR in the emergency department Radiology Service before ward admission. An upright posteroanterior chest radiograph was performed whenever possible depending on the patient's performance status and level of collaboration. In other case (i.e., severe mobility limitation and dementia), an anteroposterior supine radiograph was performed. Lateral chest radiographs were performed only in those subjects who were fully able to cooperate and maintain upright posture without assistance. CXR images were subsequently interpreted by a board-certified radiologist who was blinded to clinical data. They were also reviewed by an expert internist of the study ward at the time of admission.
Immediately after admission, a bedside LUS was performed by 1 of 3 trained internal and emergency medicine physicians with at least 1 year of certified experience in bedside ultrasonography (level 1 of training completed according the guidelines by the European Federation of Societies for Ultrasound in Medicine and Biology).[20] They were blinded to CXR results and the clinical and laboratory findings. In a randomly selected subsample of 29 patients (15 M, 14 F), LUS was independently performed by 2 physicians with the same level of skill, who were unaware of each other's findings. A Siemens Acuson X300 ultrasound system 5.0 (Siemens Healthcare GmbH, Erlangen, Germany) with a convex 2 to 5 MHz probe (Convex CH5-2 Hanafy) was used.
LUS was performed in the sitting position whenever possible. For patients with severe mobility limitations, 2 operators were involved (one performing LUS and the other helping the patient to maintain the sitting position). LUS was performed in the supine position only when forced decubitus was present (i.e., severe vertebral disease and severe trunk muscular stiffness). In this case, the bed headboard was lifted between 30 and 45 degrees for anterior-lateral scans and the patient was turned into lateral decubitus for posterior scans.
The methodology of LUS examination was consistent with current guidelines[21] and with previously published research.[22] Specifically, each hemithorax was split into anterior-lateral sectors (from parasternal to posterior axillary lines) and posterior sectors (from posterior axillary to paravertebral line). Each sector was then divided into upper and lower halves taking the third intercostal space as reference, so that 4 areas could be finally identified for each hemithorax. The probe was set perpendicular, oblique, and parallel to the ribs.
The ultrasonographic diagnosis of pneumonia was made by operators upon observation of an image of tissue-like echogenicity associated with dynamic air bronchograms, defined as punctiform or linear hyperechoic artifacts with centrifugal inspiratory dynamicity.[23] Associated abnormalities, such as pleural effusion, atelectasis, and interstitial syndrome, were diagnosed according to guidelines.[21] More specifically, atelectasis was differentiated from pneumonia by the presence of static air bronchograms, or, alternatively, no air bronchograms within the tissue-like consolidation.[23]
The final diagnosis (reference standard) was made by a skilled senior physician (the same for all patients) who reviewed the medical records integrating clinical and laboratory findings and disease course independently of LUS and CXR results. A contrast-enhanced chest CT was prescribed only in the case of persistent diagnostic uncertainty. The diagnosis was then made accordingly.
2.3. Clinical data collection
For each participant, a clinical evaluation of performance status according to the Rockwood Clinical Frailty Scale (RCFS) model was made.[19] Namely, patients were classified as managing well if they were regularly active and not dependent on others (RCFS 1–3), pre-frail if they were not dependent on others but had some mild mobility limitation such as slow gait speed (RCFS 4), and overtly frail if they were dependent on others and had severe mobility limitation including complete bedriddenness (RCFS ≥5).
The presence of the primary chronic comorbidities and laboratory data including hemoglobin, white blood cell (WBC) count, glycemia, BNP, d-dimer, high-sensitivity C-reactive protein (hs-CRP), and procalcitonin were also collected for each participant.
2.4. Statistical analyses
Data were expressed as mean ± standard deviation (SD) or, for non-normally skewed distributions, as median and interquartile range (IQR). The frequency of different lung diseases and chronic comorbidities was expressed as absolute number and percentage. The baseline characteristics of patients were compared after splitting the whole cohort according to performance status (Group 1: managing well, RCFS ≤3; Group 2: pre-frail, RCFS = 4; Group 3: frail, RCFS ≥5). Mann-Whitney's U and ANCOVA tests for multiple comparisons were used, after adjustment for age and sex.
Indeterminate or nonspecific results in both LUS and CXR were categorized as negative for the purposes of the present analysis. The diagnostic performance of LUS and CXR for pneumonia was then assessed by calculating specificity, sensitivity, negative predictive value, positive predictive value, and accuracy. Differences in these parameters were compared using the McNemar test. This analysis was first carried out on the whole cohort and then performed in subgroups after stratification according to the RCFS. In the subsample of 29 participants for which LUS was performed blindly by 2 operators, interobserver agreement was calculated.
The sample size was calculated on the basis of previously published research carried out in a different setting,[24] to highlight a 30% difference in sensitivity between LUS and CXR, with a power of 90% and an alpha risk of 0.05. The level of statistical significance was set at P ≤ 0.05. Calculations were performed using the SAS statistical package, version 9.1 (SAS Institute Inc., Cary, NC).
2.5. Ethics statement
The study protocol was approved by the local ethics committee (Comitato Etico per Parma, ID N.31842). All investigations were carried out according to the principles of the Declaration of Helsinki. All participants gave their written informed consent. For those with cognitive impairment or dementia, consent was obtained from legal representatives, according to Italian law.
3. Results
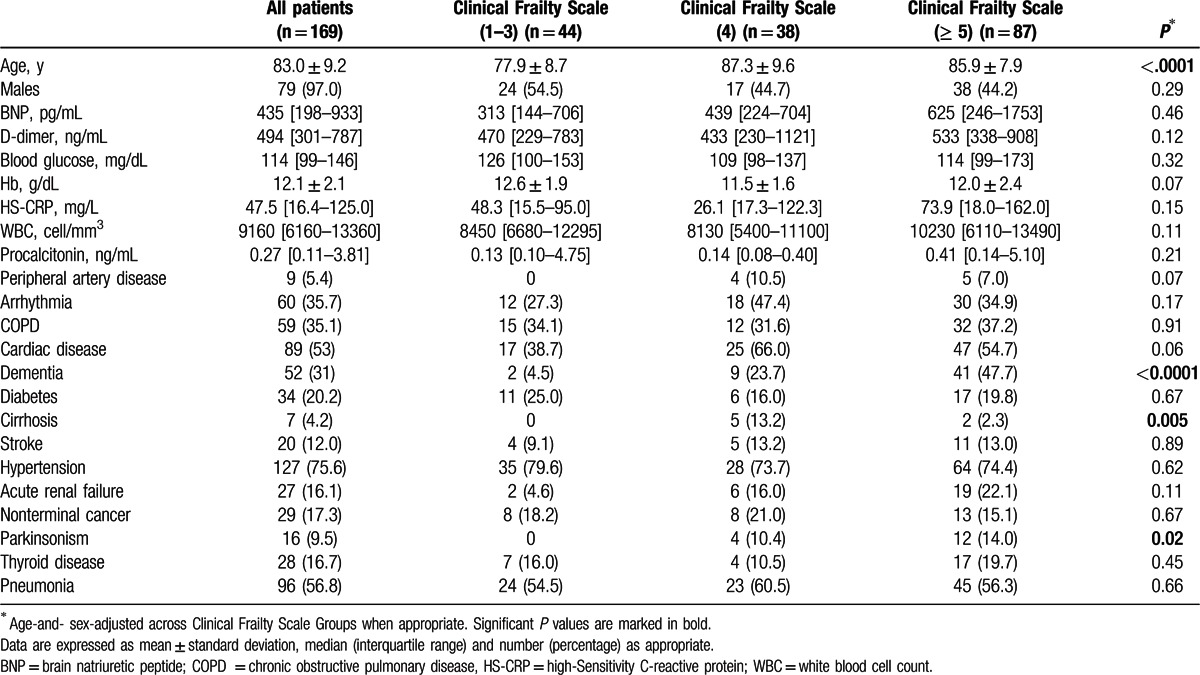
A total of 169 patients (80 M, 89 F) were included in this study, with a mean age of 83.0 ± 9.2 years. Among them, 44 (26.0%) were classified as managing well, 38 (22.5%) as pre-frail and 87 (51.5%) as frail according to the RCFS (Fig. 1). Age was significantly different across these subgroups (77.9 ± 8.7 vs 87.3 ± 9.6 vs 85.9 ± 7.9 years, P for trend < 0.001). The main clinical and laboratory features of enrolled patients at admission are summarized in Table 1. Notably, a significantly higher prevalence of dementia, Parkinsonism, and chronic liver disease was found in those with the poorest performance status. Most prevalent comorbidities were hypertension (75.6%), cardiovascular diseases (53.0%), Chronic Obstructive Pulmonary Disease (COPD) (35.1%), dementia (31.0%), and type 2 diabetes (20.2%).
Table 1.
Main clinical and laboratory characteristics of the studied population of elderly multimorbid subjects admitted to a geriatric hospital ward with acute respiratory symptoms (n = 169). Data are presented on the overall population and stratified according to the frailty status of patients, measured through Rockwood Clinical Frailty Scale.
The most frequent symptoms at presentation were dyspnea and cough, detected in 83% and 75% of patients, respectively. Only a minority of participants had pleuritic pain (17%), hemoptysis (7%), and unexplained fever with abnormal findings at chest examination (15%).
Pneumonia was diagnosed in 96 cases (56.8%), 24 in the managing well group, 23 in the prefrail group, and 45 in the frail group. The remaining 74 patients were diagnosed with acute heart failure (25 cases), acute cardiogenic pulmonary edema (21 cases), acute COPD exacerbation (16 cases), pneumothorax (4 cases), lung cancer (4 cases), pulmonary embolism (2 cases), and empyema and thoracic contusion (1 case each).
CXR was performed using an upright posteroanterior chest radiograph in 141 patients (83.4%). Among them, a lateral chest radiograph was performed only in 8 patients. The remaining 28 subjects underwent an anteroposterior supine chest radiograph.
LUS was performed in the sitting position in the majority of patients (164 of 169, 97%). The assistance of a second operator was needed in 49 cases (29%). In the remaining 5 patients, LUS was performed in the supine position and in lateral decubitus.
CXR and LUS were performed within 6 hours of each other in all participants. In this time lapse, only urgently required treatments were administered. Contrast-enhanced chest CT was necessary to clarify the diagnosis in 29 patients (17.1%). None of the patients reported adverse events or discomfort during and after diagnostic procedures.
As highlighted in Fig. 2, the presence of pneumonia was correctly identified by LUS in 88/96 cases (91%) and by CXR in 45/96 cases (47%). Accuracy, specificity, sensitivity, and negative and positive predictive values for each diagnostic examination are reported in Table 2. Notably, LUS had a significantly higher accuracy (0.90, 95% confidence interval [CI] 0.83–0.96 vs 0.67, 95% CI 0.60–0.74, P < 0.0001 with McNemar test), sensitivity (0.92, 95% CI 0.86–0.97 vs 0.47, 95% CI 0.37–0.57), and negative predictive value (0.95, 95% CI 0.83–0.96 vs 0.57, 95% CI 0.48–0.66) than CXR for diagnosing pneumonia. LUS gave a false-positive result in 3 subjects, with subsequent diagnoses of acute pulmonary embolism with infarction (2 cases) and lung cancer (1 case) by contrast-enhanced chest CT.
Figure 2.
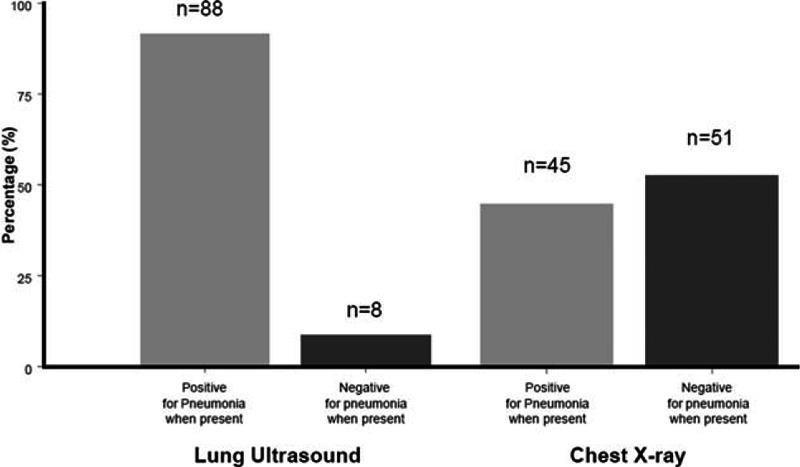
Categorization of patients with pneumonia (n = 96) obtained by lung ultrasound and chest x-ray.
Table 2.
Sensitivity, specificity, PPV, NPV, and accuracy of bedside lung ultrasound versus standard chest x-ray for identifying patients with pneumonia in the whole study population, and after stratification for Rockwood Clinical Frailty Scale.
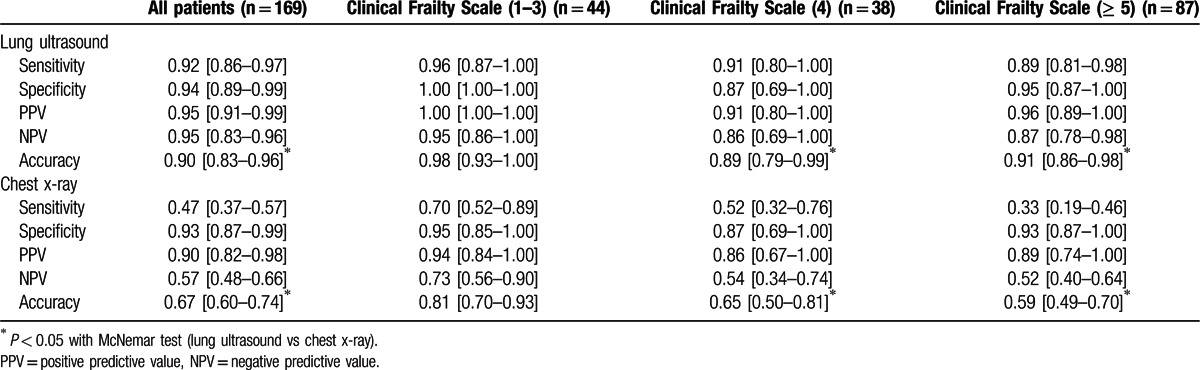
Figure 3 and Table 2 highlight the diagnostic performance of LUS and CXR after categorization of patients according to their functional performance measured through Rockwood Clinical Frailty Scale. Diagnostic accuracy of LUS and CXR was similar in those patients managing well (0.98, 95% CI 0.93–1.00 vs 0.81, 95% CI 0.70–0.93, respectively, P = 0.18 with McNemar test), but was significantly different in those with prefrailty (0.89, 95% CI 0.79–0.99 vs 0.65, 95% CI 0.50–0.81, P = 0.02) and overt frailty syndrome (0.91, 95% CI 0.86–0.98 vs 0.59, 95% CI 0.49–0.70, respectively, P = 0.0003). In this group, the specificity and negative predictive value of CXR were significantly inferior, when compared with LUS (Table 2).
Figure 3.
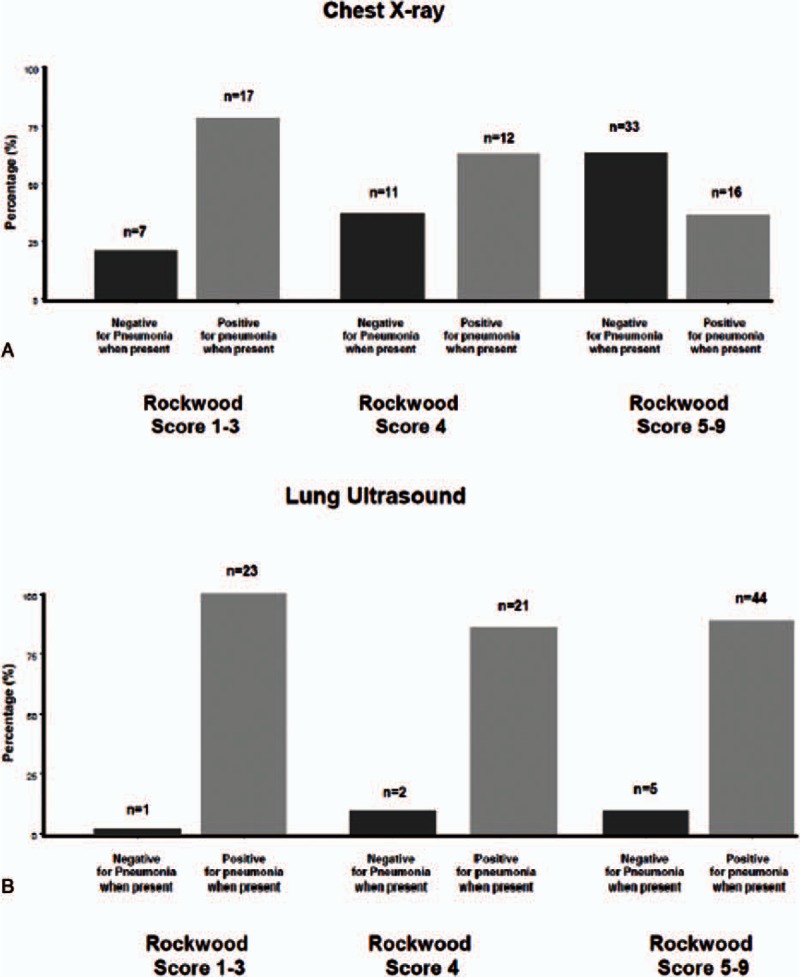
Categorization of patients with pneumonia (n = 96) obtained by chest x-ray (A) and lung ultrasound (B) across different groups of Rockwood Clinical Frailty Scale.
Interobserver agreement for LUS, calculated on a subsample of 29 participants where 2 operators performed the diagnostic test blinded to each other, was high (k = 0.90).
4. Discussion
In a cohort of elderly multimorbid patients admitted to an acute care geriatric ward with respiratory symptoms, bedside LUS proved to be significantly more accurate for the diagnosis of pneumonia than CXR. The diagnostic performance of LUS was unaffected by the frailty status of patients, while CXR showed a consistent decline in sensitivity and negative predictive value in those individuals with the highest frailty scores.
CXRs negative for consolidation in the presence of a high clinical suspicion for pneumonia are commonplace in acute-care wards.[5] Frailty and mobility limitations may have a strong impact on the diagnostic value of chest radiographs. Thus, our findings confirm previous data from the medical literature. In a study carried out on small cohort of completely bedridden individuals with suspected pneumonia, a negative CXR was proved insufficient to rule out the diagnosis.[25] The impact of mobility limitations on CXR diagnostic accuracy has been recently confirmed in adult patients with sickle-cell disease admitted to ICU for acute chest syndrome. In that observational study, bedside LUS and chest CT outperformed bedside CXR for establishing a diagnosis.[26] The accuracy of CXR was not optimal even in adult emergency department populations, thus contributing to a high number of CT prescriptions.[27,28]
In the last decade, the epidemiological features of patients admitted to acute care hospitals with suspected pneumonia have shown a trend toward complexity, with an increasing number of comorbidities.[29,30] In older individuals, multimorbidity, disability, and frailty significantly overlap.[31] Respiratory symptoms and the neurologic consequences of acute infection may significantly impact the functional abilities of seniors admitted to hospital wards, unmasking their frailty status and leading to dependency and inability to cooperate for diagnostic examinations.[32] These issues may have a strong impact on the ability of chest radiography to detect parenchymal consolidations.
The routine application of LUS in this setting may significantly improve the diagnosis. The technique is very easy to perform, not time consuming, rapidly available at the bedside and does not expose patients to ionizing radiation. Moreover, it can be easily learned by clinicians with a standard level of ability to handle an ultrasound probe for abdominal scans.
Several studies have demonstrated the superiority of LUS performed by emergency department physicians over standard CXR in the differential diagnosis of acute respiratory symptoms.[10,22,24,33–37] This technique can provide reliable diagnostic information even when performed by trained nurses in a busy emergency department setting, in the presence of a specific diagnostic question.[38] Other studies have investigated the diagnostic performance of LUS in an internal medicine ward setting,[11,12] but none of them have focused specifically on an elderly population considering functional status in multimorbid participants.
LUS has also been demonstrated to be highly accurate in the detection of parenchymal consolidation in patients with acute respiratory failure admitted to ICUs.[39,40] The diagnostic performance of LUS is in fact superior than that of CXR in this setting,[41] and even comparable to that of contrast-enhanced CT in its ability to detect pulmonary edema, asthma, and COPD, and to raise the clinical suspicion of pulmonary embolism.[42–44] The use of this technique is also supported by rapidity and probably by cheapness, although no studies have investigated the cost-effectiveness of LUS implementation in medical wards to date.
It is noteworthy that only a few CT scans (17.1%) were performed to clarify the diagnosis in our study. Given the high diagnostic accuracy of LUS in this setting, we hypothesize that ultrasonography may help to reduce the number of chest CTs and improve the appropriateness of their prescription.
We must, however, acknowledge that the lack of CT scans, as reference standard test for all participants is the main limitation of our study. To date, the diagnostic reference standard was set by chest CT of all participants in only a few studies comparing CXR versus LUS.[26,45] In other cases, different methodologies for obtaining a gold standard reference were used, including chest CT prescription only in those with a negative CXR[11] and masked audit, that is, retrospective review of medical records blinded to imaging results.[10]
Moreover, another potential source of bias in our study was the nonsimultaneous timing of execution of CXR and LUS. However, both of these studies were performed within 6 hours from each other, as was the procedure in other related studies.[10] Additional limitations include the relatively small sample size and the high pretest probability of pneumonia in the studied population. Moreover, the actual diagnostic performance of CXR could have been underestimated, due to the enrolment of a high number of subjects with disability. Finally, the examinations were performed by trained medical physicians with few years of experience in clinical ultrasonography, and not by expert certified lung sonographers. However, in a recent study carried out in an Italian emergency department population, LUS was performed in some cases by both inexperienced residents and experienced senior physicians, with a good interobserver agreement.[22] Thus, as also suggested by the findings of the present study, the diagnostic performance of LUS might be only marginally affected by the level of skill of operators, provided that they are confident with general ultrasound technique and semeiotics.
The simplicity and reproducibility of the protocol may represent the main strength of this study. Since the diagnosis of pneumonia in elderly multimorbid individuals is often challenging, LUS could be introduced in various settings of geriatric care and integrated with other screening tools for pneumonia detection. More specifically, bedside LUS could be recommended in those older patients with poor functional performance and high clinical suspicion of pneumonia, in the presence of a negative chest radiograph. In this situation, the ultrasound results could help clarify the diagnosis and avoid chest CT prescription. However, we acknowledge that a wide application of this algorithm will need confirmation of our findings in larger studies, comparing the LUS results also with CT in all participants. CXR will remain the standard first-level diagnostic test until that. Moreover, ultrasonographic skills are not uniform among geriatricians and internal medicine specialists across different hospitals and countries. This represents a further limitation to the applicability of the algorithm proposed earlier, although LUS is a relatively simple technique that can be effectively learned by training physicians.
5. Conclusions
In elderly multimorbid patients admitted to an acute-care ward with a high clinical suspicion of pneumonia, bedside lung ultrasonography is an easy, rapid, safe, and accurate tool to confirm or rule out this diagnosis. Unlike chest radiography, its accuracy is not affected by the performance status of patients.
Acknowledgments
The authors wish to thank all patients who participated to the study and Drs Marco Davìd Zani and Nicoletta Cerundolo for the important contribution in bedside ultrasonography implementation in the Geriatric-Rehabilitation Department of Parma University Hospital. Both Drs Zani and Cerundolo gave permission to be named.
Footnotes
Abbreviations: CXR = chest x-ray, ICU = intensive care unit, LUS = lung ultrasonography, RCFS = Rockwood Clinical Frailty Scale.
Preliminary data of this study were presented at the 116th National Congress of the Italian Society of Internal Medicine (S.I.M.I.) held in Rome from October 10 to 12, 2015, and were awarded the best oral communication prize in the “clinical ultrasound” section.
AT, AN, MM, and TM did the study design; FL did the statistical analyses; AN, GM, and GC did the data acquisition and interpretation; AT did the manuscript writing; FL, MM, and TM did the critical review of the manuscript for important intellectual content.
This work was supported by an unconditional grant from the Cariparma Foundation (ID no. 2014-0186), www.fondazionecrp.it (Italian language). The funder had no role in study design and realization. Data were collected, analyzed, and interpreted by independent researchers with no financial relationship with the funding source.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Chavez MA, Shams N, Ellington LE, et al. Lung ultrasound for the diagnosis of pneumonia in adults: a systematic review and meta-analysis. Resp Res 2014; 15:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu QJ, Shen YC, Jia LQ, et al. Diagnostic performance of lung ultrasound in the diagnosis of pneumonia: a bivariate meta-analysis. Int J Clin Exp Med 2014; 7:115–121. [PMC free article] [PubMed] [Google Scholar]
- 3.Ye X, Xiao H, Chen B, et al. Accuracy of lung ultrasonography versus chest radiography for the diagnosis of adult community-acquired pneumonia: review of the literature and meta-analysis. PLoS One 2015; 10:e0130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albaum MN, Hill LC, Murphy M, et al. Interobserver reliability of the chest radiograph in community-acquired pneumonia. Chest 1996; 110:343–350. [DOI] [PubMed] [Google Scholar]
- 5.Basi SK, Marrie TJ, Huang JQ, et al. Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med 2004; 117:305–311. [DOI] [PubMed] [Google Scholar]
- 6.Reissig A, Gramegna A, Aliberti S. The role of lung ultrasound in the diagnosis and follow-up of community-acquired pneumonia. Eur J Intern Med 2012; 23:391–397. [DOI] [PubMed] [Google Scholar]
- 7.Lichtenstein DA. BLUE-protocol and FALLS-protocol. Two applications of lung ultrasound in the critically ill. Chest 2015; 147:1659–1670. [DOI] [PubMed] [Google Scholar]
- 8.Zanobetti M, Poggioni C, Pini R. Can chest ultrasonography replace standard chest radiography for evaluation of acute dyspnea in the ED? Chest 2011; 139:1140–1147. [DOI] [PubMed] [Google Scholar]
- 9.Peris A, Tutino L, Zagli G, et al. The use of point-of-care bedside lung ultrasound significantly reduces the number of radiographs and computed tomography scans in critically ill patients. Anesth Analg 2010; 111:687–692. [DOI] [PubMed] [Google Scholar]
- 10.Laursen CB, Sloth E, Lassen AT, et al. Point-of-care ultrasonography in patients admitted with respiratory symptoms: a single-blind, randomised controlled trial. Lancet Resp Med 2014; 2:638–646. [DOI] [PubMed] [Google Scholar]
- 11.Reissig A, Copetti R, Mathis G, et al. Lung ultrasound in the diagnosis and follow-up of community-acquired pneumonia. A prospective, multicenter, diagnostic accuracy study. Chest 2012; 142:965–972. [DOI] [PubMed] [Google Scholar]
- 12.Sperandeo M, Carnevale V, Muscarella S, et al. Clinical application of transthoracic ultrasonography in inpatients with pneumonia. Eur J Clin Invest 2011; 41:1–7. [DOI] [PubMed] [Google Scholar]
- 13.Torres A, Peetermans WE, Viegi G, et al. Risk factors for community-acquired pneumonia in adults in Europe: a literature review. Thorax 2013; 68:1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faverio P, Aliberti S, Bellelli G, et al. The management of community-acquired pneumonia in the elderly. Eur J Intern Med 2014; 25:312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soong J, Poots AJ, Scott S, et al. Quantifying the prevalence of frailty in English hospitals. BMJ Open 2015; 5:e008456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown RT, Pierluissi E, Guzman D, et al. Functional disability in late-middle-aged and older adults admitted to a safety-net hospital. J Am Geriatr Soc 2014; 62:2056–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Timmons S, Manning E, Barrett A, et al. Dementia in older people admitted to hospital: a regional multi-hospital observational study of prevalence, associations and case recognition. Age Ageing 2015; 44:993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arienti V, Camaggi V. Clinical applications of bedside ultrasonography in internal and emergency medicine. Intern Emerg Med 2011; 6:195–201. [DOI] [PubMed] [Google Scholar]
- 19.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.European Federation of Societies for Ultrasound in Medicine Biology. Minimum training recommendations for the practice of medical ultrasound. Ultraschall Med 2006; 27:79–105. [DOI] [PubMed] [Google Scholar]
- 21.Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 2012; 38:577–591. [DOI] [PubMed] [Google Scholar]
- 22.Nazerian P, Volpicelli G, Vanni S, et al. Accuracy of lung ultrasound for the diagnosis of consolidations when compared to chest computed tomography. Am J Emerg Med 2015; 33:620–625. [DOI] [PubMed] [Google Scholar]
- 23.Lichtenstein D, Mezière G, Seitz J. The dynamic air bronchogram. A lung ultrasound sign of alveolar consolidation ruling out atelectasis. Chest 2009; 135:1421–1425. [DOI] [PubMed] [Google Scholar]
- 24.Pagano A, Numis FG, Visone G, et al. Lung ultrasound for diagnosis of pneumonia in emergency department. Intern Emerg Med 2015; 10:851–854. [DOI] [PubMed] [Google Scholar]
- 25.Esayag Y, Nikitin I, Bar-Ziv J, et al. Diagnostic value of chest radiographs in bedridden patients suspected of having pneumonia. Am J Med 2010; 123: 88.e1-88.e6. [DOI] [PubMed] [Google Scholar]
- 26.Razazi K, Deux JF, de Prost N, et al. Bedside lung ultrasound during acute chest syndrome in sickle cell disease. Medicine 2016; 95:e2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayden GE, Wrenn KW. Chest radiograph vs computed tomography scan in the evaluation for pneumonia. J Emerg Med 2009; 36:266–270. [DOI] [PubMed] [Google Scholar]
- 28.Self WH, Courtney DM, McNaughton CD, et al. High discordance of chest x-ray and computed tomography for detection of pulmonary opacities in ED patients: implications for diagnosing pneumonia. Am J Emerg Med 2013; 31:401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JS, Nsa W, Hausmann LRM, et al. Quality of care for elderly patients hospitalized for pneumonia in the United States, 2006 to 2010. JAMA Intern Med 2014; 174:1806–1814. [DOI] [PubMed] [Google Scholar]
- 30.Ticinesi A, Nouvenne A, Folesani G, et al. An investigation of multimorbidity measures as risk factors for pneumonia in elderly frail patients admitted to hospital. Eur J Intern Med 2016; 28:102–106. [DOI] [PubMed] [Google Scholar]
- 31.Theou O, Rockwood MRH, Mitnitski A, Rockwood K. Disability and co-morbidity in relation to frailty: how much do they overlap? Arch Gerontol Geriatr 2012; 55:e1–e8. [DOI] [PubMed] [Google Scholar]
- 32.Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet 2013; 381:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volpicelli G, Caramello V, Cardinale L, et al. Diagnosis of radio-occult pulmonary conditions by real-time chest ultrasonography in patients with pleuritic pain. Ultrasound Med Biol 2008; 34:1717–1723. [DOI] [PubMed] [Google Scholar]
- 34.Parlamento S, Copetti R, Di Bartolomeo S. Evaluation of lung ultrasound for the diagnosis of pneumonia in the ED. Am J Emerg Med 2009; 27:379–384. [DOI] [PubMed] [Google Scholar]
- 35.Cortellaro F, Colombo S, Coen D, et al. Lung ultrasound is an accurate diagnostic tool for the diagnosis of pneumonia in the emergency department. Emerg Med J 2012; 29:19–23. [DOI] [PubMed] [Google Scholar]
- 36.Gallard E, Redonnet JP, Bourcier JE, et al. Diagnostic performance of cardiopulmonary ultrasound performed by the emergency physician in the management of acute dyspnea. Am J Emerg Med 2015; 33:352–358. [DOI] [PubMed] [Google Scholar]
- 37.Bourcier JE, Paquet J, Seinger M, et al. Performance comparison of lung ultrasound and chest x-ray for the diagnosis of pneumonia in the ED. Am J Emerg Med 2014; 32:115–118. [DOI] [PubMed] [Google Scholar]
- 38.Mumoli N, Vitale J, Giorgi-Pierfranceschi M, et al. Accuracy of nurse-performed lung ultrasound in patients with acute dyspnea. A prospective observational study. Medicine 2016; 95:e2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lichtenstein DA, Lascols N, Mezière G, et al. Ultrasound diagnosis of alveolar consolidation in the critically ill. Intensive Care Med 2004; 30:276–281. [DOI] [PubMed] [Google Scholar]
- 40.Hew M, Corcoran JP, Harriss EK, et al. The diagnostic accuracy of chest ultrasound for CT-detected radiographic consolidation in hospitalised adults with acute respiratory failure: a systematic review. BMJ Open 2015; 5:e007838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xirouchaki N, Magkanas E, Vaporidi K, et al. Lung ultrasound in critically ill patients: comparison with bedside chest radiography. Intensive Care Med 2011; 37:1488–1493. [DOI] [PubMed] [Google Scholar]
- 42.Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure. The BLUE Protocol. Chest 2008; 134:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koenig S, Chandra S, Alaverdian A, et al. Ultrasound assessment of pulmonary embolism in patients receiving CT pulmonary angiography. Chest 2014; 145:818–823. [DOI] [PubMed] [Google Scholar]
- 44.Sekiguchi H, Schenck LA, Horie R, et al. Critical care ultrasonography differentiates ARDS, pulmonary edema, and other causes in the early course of acute hypoxemic respiratory failure. Chest 2015; 148:912–918. [DOI] [PubMed] [Google Scholar]
- 45.Liu X, Lian R, Tao Y, et al. Lung ultrasonography: an effective way to diagnose community-acquired pneumonia. Emerg Med J 2015; 32:433–438. [DOI] [PubMed] [Google Scholar]


