Abstract
Accumulating evidence indicates that mucin antigen MUC1 plays a fundamental role in the initiation and progression of several types of epithelial carcinomas. However, whether the expression of MUC1 on tumor cells is associated with patients’ survival remains controversial.
Medline/PubMed, EMBASE, the Cochrane Library, Chinese National Knowledge Infrastructure (CNKI) databases, and Grey literature were searched up to 15 August 2015 for eligible studies of the association between the MUC1 expression and overall survival (OS) in various epithelial cancers. The hazard ratio (HR) and its 95% confidence interval (CI) were calculated from the included studies. Moreover, the odds ratio (OR) was also extracted to evaluate the association between the clinicopathological parameters of participants and MUC1 expression.
A total of 3425 patients covering 23 studies were included in the analysis. The pooled results showed that positive MUC1 staining was a negative predictor of OS (HRFEM = 1.98,95% CIFEM: 1.76–2.22, PFEM = 0.479; HRREM = 2.16,95% CIREM: 1.58–2.94, PREM = 0.355) in various epithelial carcinomas. Subgroup analysis revealed that the increased MUC1 expression was significantly associated with poor OS in patients with gastric cancer (HRFEM = 2.12, 95%CIFEM: 1.75–2.57, PFEM = 0.359; HRREM = 1.89, 95% CIREM: 1.05–3.41, PREM = 0.238), colorectal cancer (HRFEM = 1.73, 95%CIFEM: 1.41–2.13, PFEM = 0.048; HRREM = 2.00,95% CIREM: 1.46–2.73, PREM = 0.019), cholangiocarcinoma (HRFEM = 2.52, 95% CIFEM: 1.42–4.49, PFEM = 0.252; HRREM = 2.34, 95% CIREM: 1.30–4.22, PREM = 0.244), and nonsmall cell lung cancer (NSCLC) (HRFEM = 2.14, 95% CIFEM: 1.46–3.14, PFEM = 0.591; HRREM = 2.81, 95% CIREM: 1.40–5.64, PREM = 0.280). In addition, MUC1 overexpression was more likely to be found in colorectal cancer patients with an advanced tumor node metastasis stage (ORREM = 1.55, 95% CIREM: 1.06–2.27; PREM = 0.187) and in gastric cancer patients with positive lymph node metastasis (ORREM = 2.37, 95% CIREM: 1.19–4.73; PREM = 0.004) and intestinal-type classification (ORREM = 2.34, 95% CIREM: 1.59–3.45; PREM = 0.767).
Our findings provide evidence that MUC1 detection has a prognostic value in patients with epithelial-originated cancers, especially in NSCLC and gastrointestinal cancers.
INTRODUCTION
Cancer is currently the second leading cause of death in the United States and is a major public health problem worldwide. In 2015, ∼1658,370 new cases will be diagnosed with cancer, with an estimated 589,430 deaths.1 Although cancer death rates have been declining for the past 2 decades, there are no suitable biomarkers for predicting tumor progression, tumor metastasis, and response to treatment.2 Thus, it is still a great need to detect better predictors for clinical application, especially for predicting the outcome of cancer patients.
Mucins are a family of highly glycosylated proteins with a basic structure consisting of a variable number of tandem repeats rich in serine, threonine, and proline. Biochemically, mucins comprise a large gene family of ∼20 molecules, which can broadly be divided into secreted and membrane-associated mucins. Among them, Mucin 1 (MUC1), a transmembrane glycoprotein that is normally expressed at the basal level in human epithelial cells to provide a protective barrier for the epithelial cells,3 has been the focus of research. In neoplastic cells, MUC1 expression can be upregulated to stimulate tumor cell proliferation and also suppress apoptosis, which supports a role for MUC1 in tumor progression.4 In addition, it has been reported that MUC1 could reduce the cell–cell adhesion to the extracellular matrix,5 which would lead to an increased invasive and metastatic capability of tumor cells. In vivo experiment of transgenic mice demonstrated that overexpression of MUC1 in mouse mammary tissue results in spontaneous mammary gland carcinoma.6 Furthermore, MUC1 in tumor cells is aberrantly glycosylated, which resulted in overexpression of several novel tumor-associated carbohydrate structures such as Tn antigen, T antigen, and sialyl-Tn antigen, suggesting a possible prognostic role in diverse kinds of human cancers.7 Taken together, MUC1 may play a role in tumorigenesis, progression, and metastasis and thus may serve as a potential prognostic factor of epithelial-originated cancer.
Moreover, some researchers have investigated MUC1 expression by immunohistochemical (IHC) staining in several human epithelial malignancies including gastric,8 colorectal,9 pancreatic,10 breast,11 endometrial,12 lung,13 bladder,14 and renal cell cancers.15 However, its prognostic role in cancers is still under debate. In this study, we aimed to perform an up-to-date meta-analysis to reveal the prognostic value of MUC1 in various human epithelial carcinomas.
METHOD
Search Strategy
We searched several international databases including Medline/PubMed, EMBASE, the Cochrane Library, and Chinese National Knowledge Infrastructure (CNKI) databases up to 15 August 2015. The key terms used for literature retrieval included “Mucin1” or “MUC1” or “Episialin” and “cancer” or “carcinoma” or “tumor” or “neoplasm” and “survival” or “outcome” or “prognosis.” To obtain additional relevant manuscripts, conference summaries and reference lists missed in the retrieval were identified. We even contacted the corresponding authors to get additional information if necessary. All procedures were approved by the Ethics Committee for human experiments of Capital Medical University.
Selection Criteria
Studies were selected if they met the following criteria: (a) they focused on epithelial cancers; (b) all selected cancer patients were pathologically confirmed; (c) the expression level of MUC1 was tested by immunohistochemistry staining; and (d) correlation between MUC1, clinicopathological features, and overall survival was described.
Articles were excluded from the analyses based on the following criteria: (a) non-English papers; (b) nonhuman experiments; (c) review articles, case reports, or letters; (d) duplicate publication; and (e) insufficient data to report the hazard ratios (HR) and 95% confidence interval (95% CI), or the Kaplan–Meier curve could not be extracted.
Data Extraction
All relevant data were screened and extracted by 2 independent investigators (FX and GSF). The quality of the selected articles was assessed according to the Newcastle–Ottawa Scale (NOS).15 The information including author, year of publication, country, patient number, cancer type, the cut-off value, overexpression rates of MUC1, antibody for MUC1 staining, hazard ratio, and follow-up time was recorded for each study. To get the HRs and their 95%CI that were not reported in the articles, we digitized and extracted the data from the Kaplan–Meier curves using the software designed by Jayne F Tierney and Matthew R Sydes.16 To reach a consensus, any disagreement on a conflicting study was resolved by full discussion.
Statistical Analysis
The statistical analysis was performed according to the guidelines proposed by the Meta-Analysis of Observational Studies in Epidemiology group (MOOSE).17 Hazard ratio with 95% CI was calculated using Stata SE12.0 (Stata Corporation, TX). The heterogeneity among studies was measured using the Q and I2 tests. A probability value of P < 0.1 and I2 ≥ 50% indicated the existence of significant heterogeneity.18 Both the fixed-effect model (FEM) and random-effect model (REM) were used to estimate the association between MUC1 expression and OS.19 The potential for publication bias was assessed using Begg's funnel plot and the Egger linear regression test. P value < 0.05 was considered statistically significant. All P values are 2-tailed.
RESULTS
Search Results
The initial search returned a total of 119 manuscripts. After screening the titles and abstracts, 96 irrelevant articles were excluded according to the search strategy above. As a result, a total of 23 studies were included in our meta-analysis. The detailed screening process was shown in Figure 1. All of these enrolled studies comprehensively assessed the expression of MUC1 and the OS rate.
FIGURE 1.
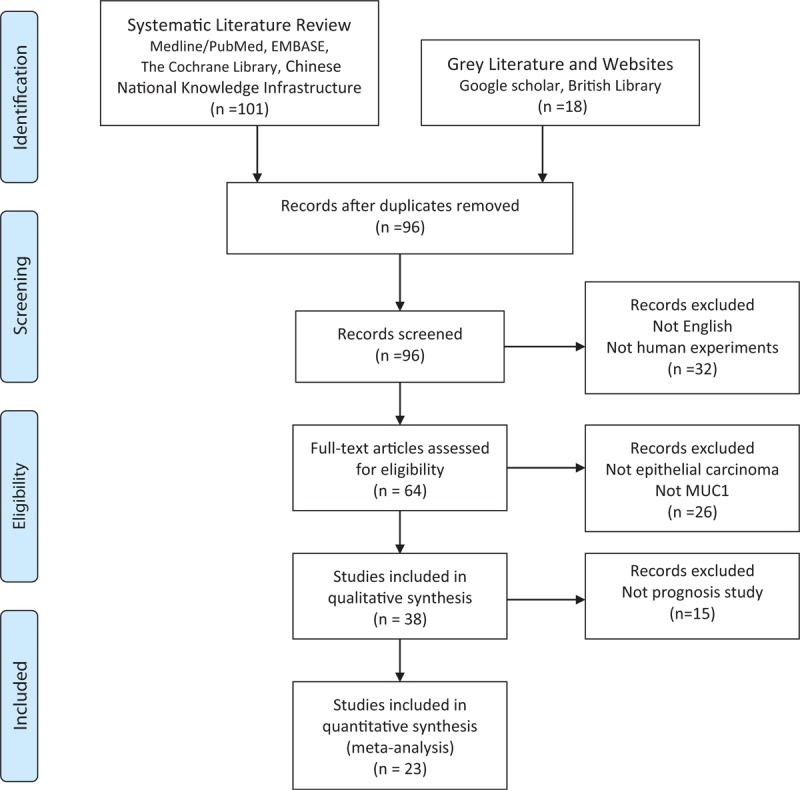
PRISMA flowchart of the literature search.
Study Selection and Characteristics
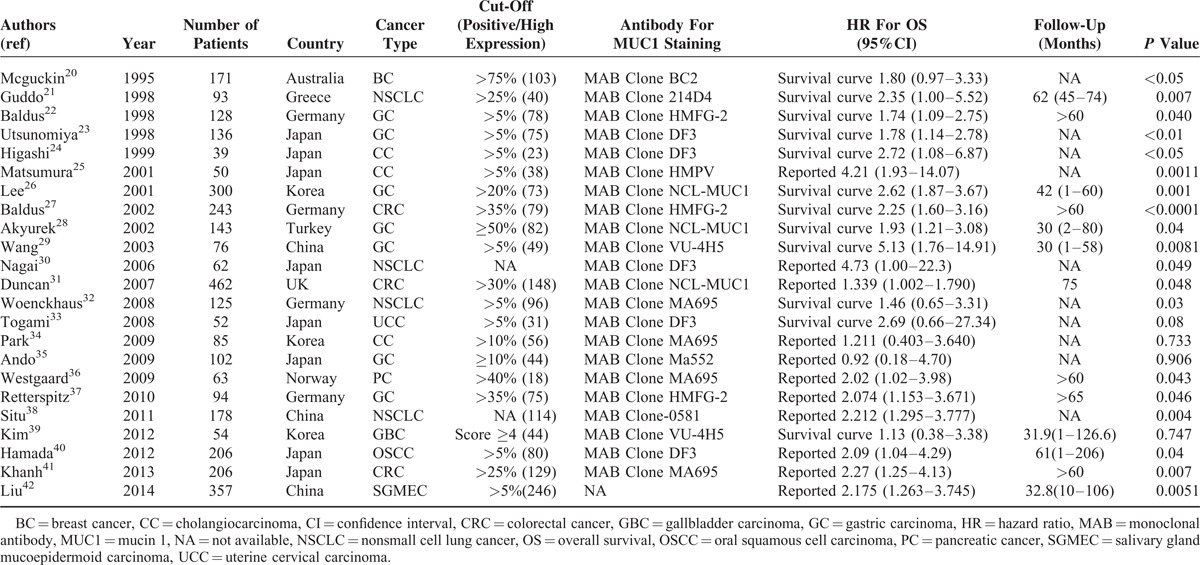
All features of the 23 eligible studies are listed in Table 1.20–42 Among them, participants in 14 studies were Asian and in the other 9 were Caucasian. Our study focused on 10 types of epithelial malignancies, including breast cancer (BC), cholangiocarcinoma (CC), uterine cervical carcinoma (UCC), colorectal cancer (CRC), oral squamous cell carcinoma (OSCC), gastric carcinoma (GC), gallbladder carcinoma (GBC), nonsmall cell lung cancer (NSCLC), pancreatic cancer (PC), and salivary gland mucoepidermoid carcinoma (SGMEC). The cut-off values of IHC evaluation applied in the studies were not consistent ranging from 5% to 75%. Regarding different anti-MUC1 monoclonal antibodies, 3 studies used clone HMFG-2, 5 for clone DF3, 3 for clone NCL-MUC1, 2 for clone VU-4H5, 4 for clone MA695, and 6 focused on others. Hazard ratios with their 95% CIs were extracted from the graphical survival plots in 12 studies and reported directly in 11 studies, 15 of which calculated HRs by the multivariate analysis and 8 via univariable analysis. In all studies, none of the patients received neo-adjuvant radio- or chemotherapy prior to surgery.
TABLE 1.
Characteristics of Included Studies for Meta-Analyses
Correlation of MUC1 Expression With Prognosis
As shown in Table 2, we found that elevated MUC1 predicted a poor outcome (HRFEM = 1.98, 95% CIFEM: 1.76–2.22, PFEM = 0.479; HRFEM = 2.16, 95% CIFEM: 1.58–2.94, PFEM = 0.355) for 23 studies evaluating OS (Figs. 2 and 3). Regarding diverse cancer types, subgroup analyses using a fixed effect model showed that increased MUC1 predicted an unfavorable prognosis in gastric cancer (HRFEM = 2.12, 95%CIFEM: 1.75–2.57, PFEM = 0.359), colorectal cancer (HRFEM = 1.73, 95%CIFEM: 1.41–2.13, PFEM = 0.048), cholangiocarcinoma (HRFEM = 2.52, 95% CIFEM: 1.42–4.49, PFEM = 0.252), and nonsmall cell lung cancer (HRFEM = 2.14, 95% CIFEM: 1.46–3.14, PFEM = 0.591). When ethnicity was considered, results revealed that positive MUC1 expression indicating poor OS was found both in Asian people (HRFEM = 2.28, 95%CIFEM: 1.90–2.72, PFEM = 0.683) and Caucasian populations (HRFEM = 1.77, 95%CIFEM: 1.51–2.07, PFEM = 0.678). When performing subgroup analyses stratified by the analysis method, it is found that increased MUC1 was a negative predictor for OS both by univariable analysis (HRFEM = 2.05, 95%CIFEM: 1.70–2.47, PFEM = 0.694) and multivariable analysis (HRFEM = 2.00, 95%CIFEM: 1.67–2.39, PFEM = 0.271). Considering different anti-MUC1 monoclonal antibodies, MUC1 was a negative prognostic marker for the data applying clone HMFG-2 (HRFEM = 2.06, 95%CIFEM: 1.61–2.64, PFEM = 0.680), clone DF3 (HRFEM = 2.07, 95%CIFEM: 1.48–2.89, PFEM = 0.747), clone NCL-MUC1 (HRFEM = 1.81, 95%CIFEM: 1.48–2.21, PFEM = 0.012), clone VU-4H5 (HRFEM = 2.45, 95%CIFEM: 1.14–5.26, PFEM = 0.052), clone MA695 (HRFEM = 1.86, 95%CIFEM: 1.29–2.70, PFEM = 0.704), and other antibodies (HRFEM = 2.18, 95%CIFEM: 1.64–2.90, PFEM = 0.675). Furthermore, subgroup analyses utilizing the random effect model showed consistent results in Table 2.
TABLE 2.
Meta Analysis Results
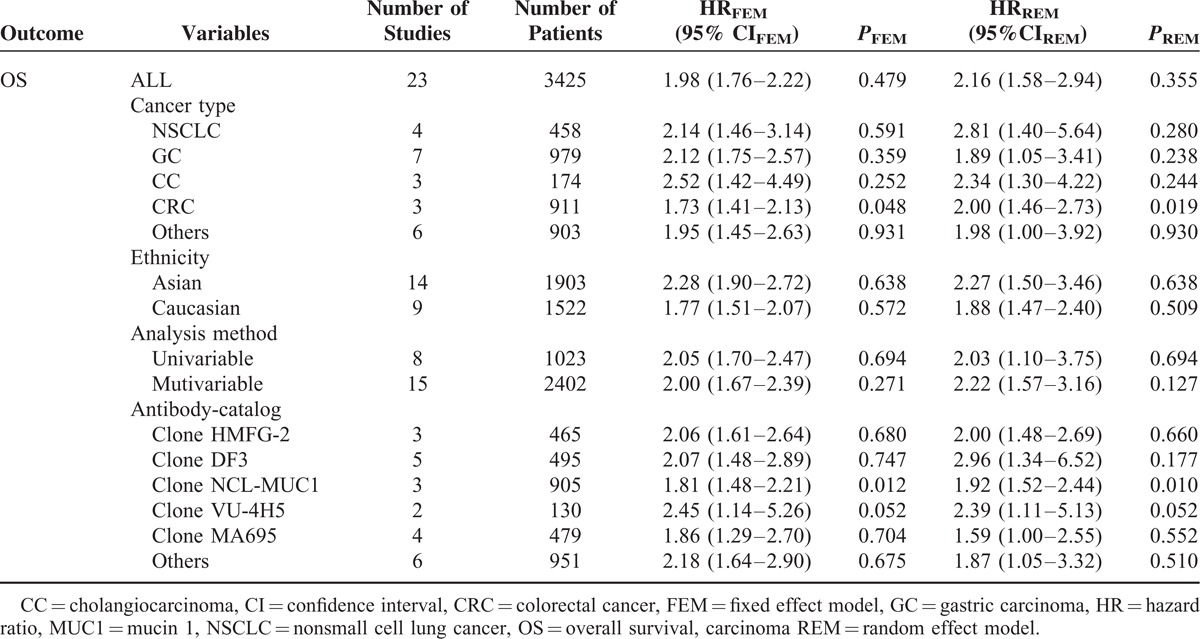
FIGURE 2.
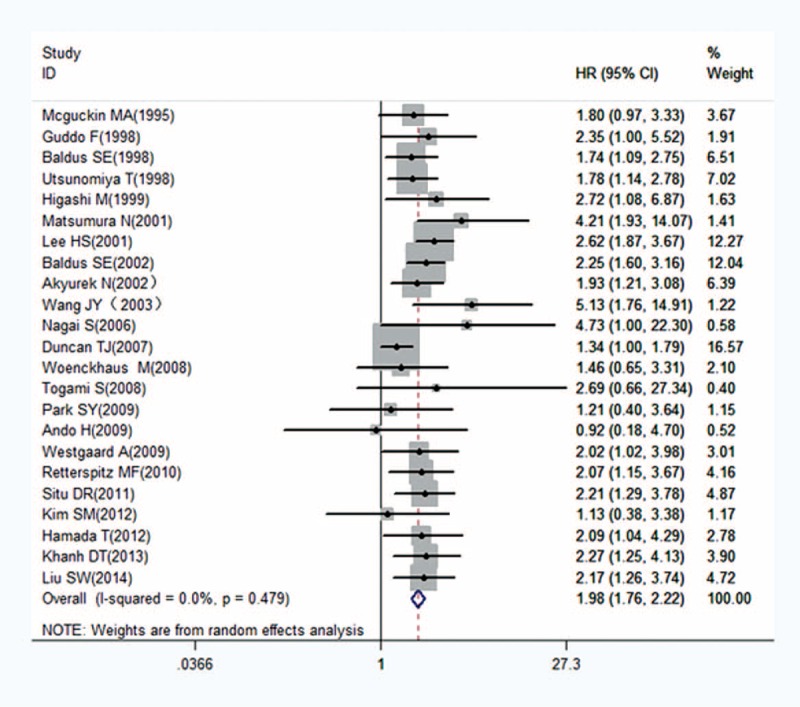
Forest plots of studies evaluating hazard ratios (HRs) of MUC1 for overall survival with a fixed effect model. HRs = hazard ratios, MUC1 = mucin 1.
FIGURE 3.
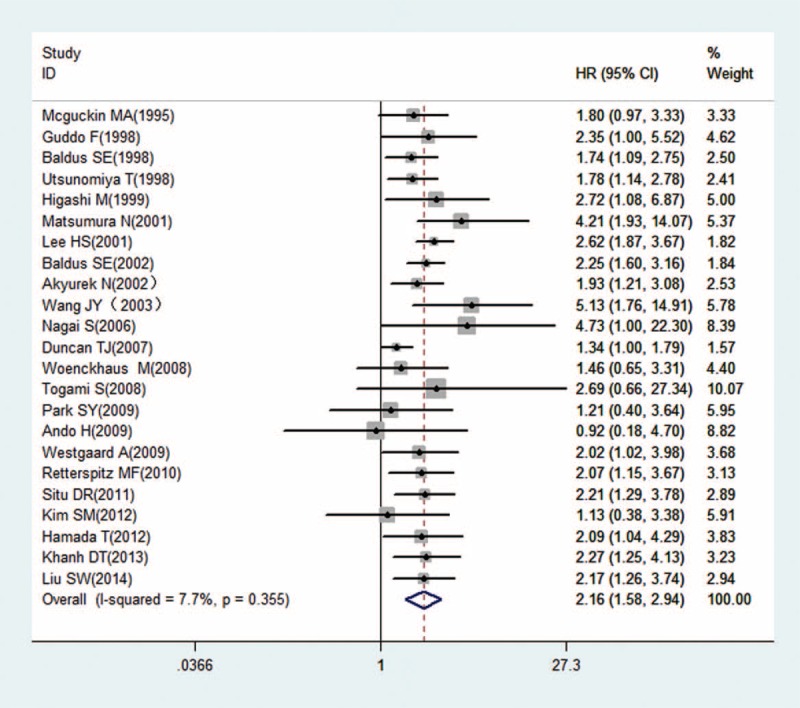
Forest plots of studies evaluating hazard ratios (HRs) of MUC1 for overall survival with a random effect model. HRs = hazard ratios, MUC1 = mucin 1.
Correlation of MUC1 Expression With Clinicopathological Features
The relationship between elevated MUC1 and clinicopathological parameters (reported in at least 3 studies) was explored with a random effect model in colorectal cancer and gastric cancer. As shown in Figure 4, increased MUC1 was found to be significantly associated with positive lymph node metastasis and intestinal-type classification but not with advanced tumor node metastasis (TNM) stage in gastric cancer. In addition, MUC1 overexpression was more likely to be found in colorectal cancer patients with an advanced TNM stage. However, no significant relationship was detected between MUC1 overexpression and clinical characteristics in other epithelial cancers due to limited studies.
FIGURE 4.
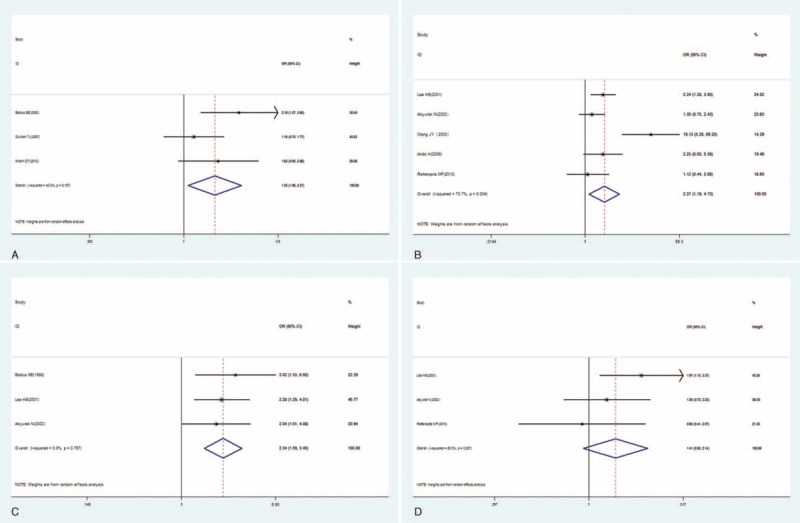
Forest plots of studies evaluating the association between MUC1 and clinical parameters in CRC and GC with a random effect model. (A) Tumor node metastasis (TNM) stage (advanced vs early) in CRC; (B) lymph node metastasis (present vs absent) in GC; (C) Lauren classification (intestinal-type vs diffuse-type) in GC; (D) TNM stage (advanced vs early) in GC. CRC = colorectal cancer, GC = gastric carcinoma, MUC1 = mucin 1.
Heterogeneity
To explore the potential source of heterogeneity among studies, “metareg” STATA command was conducted utilizing variables as year of publication, ethnicity (Asian vs Caucasian), cancer type, and analysis method (univariable vs mutivariable). The results showed that no variable included in the meta-regression contributed to the heterogeneity.
Sensitivity Analysis
The selected studies were sequentially removed to investigate whether any single study could have an influence on the pooled results. As shown in Figure 5, the stable pooled HRs was found to be not significantly affected by each individual study.
FIGURE 5.
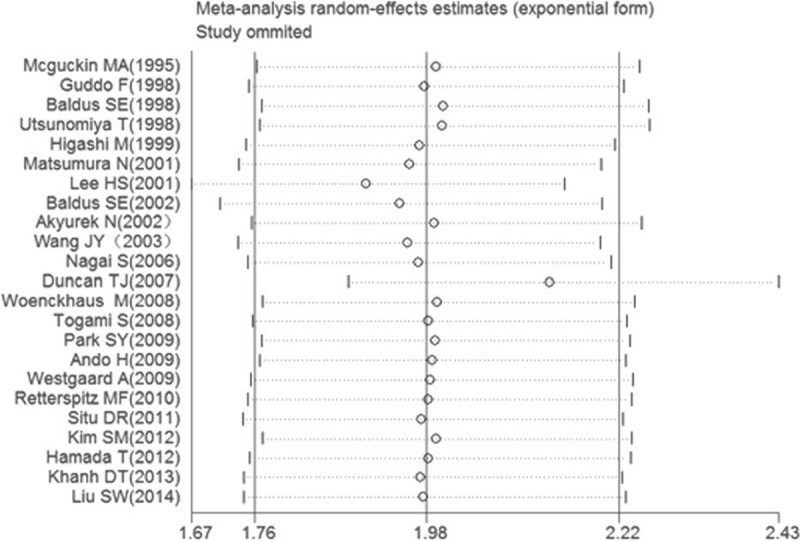
Effect of individual studies on the pooled HR for MUC1 and OS of patients. HR = hazard ratio, MUC1 = mucin 1, OS = overall survival.
Publication Bias
Bgger's funnel plot (Fig. 6) was almost symmetric and the P value of Egger's linear regression test was 0.296. Thus, no evidence for publication bias in this meta-analysis was found.
FIGURE 6.
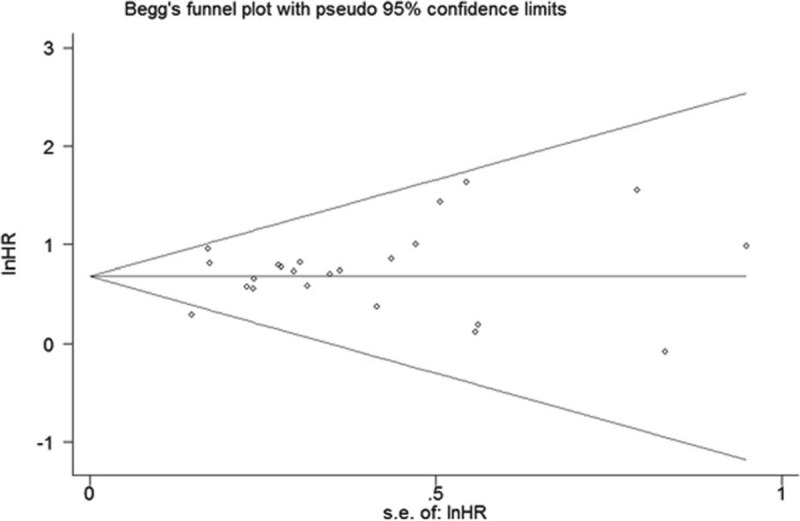
Begg's funnel plots for all of the included studies reported with OS. OS = overall survival.
DISCUSSION
To date, the relationship between MUC1 expression and the outcome of tumor sufferers remains inconclusive. Our current study chiefly concerned with the prognostic role of MUC1 in epithelial-originated cancer. To the best of our knowledge, it is the first meta-analysis to investigate the prognostic role of MUC1 in various human epithelial cancers. We found that positive/higher MUC1 expression significantly predicted poorer OS compared with negative/lower MUC1 expression. The pooled results indicated that MUC1 might act as a reliable biomarker in predicting clinical outcomes of human epithelial cancers.
The mechanisms responsible for the above association derived the following interpretations. As a common feature of epithelial-originated tumors, overexpression of MUC1 is caused by the genetic and transcriptional levels of gene expression control and by a loss of post-transcriptional regulation. Amplification of the MUC1 gene locus (1q21) has been observed in breast cancer cells.43 Through associations with various transcription factors (STAT3, NF-кB, p53, and β-catenin), MUC1 could upregulate its own promoter activity.44 Moreover, MUC1 overexpression is regulated post-transcriptionally through microRNA-125b targeting the 3′untranslated region (UTR) of MUC1 mRNA in breast cancer cells.45 Several studies have indicated that MUC1 plays a critical role in tumor invasion and metastasis. On one hand, MUC1 involves in the biological process of epithelial to mesenchymal transition (EMT) by repressing E-cadherin expression and upregulating expression of several EMT inducers,46 by which tumor cells acquire their invasive potential. On the other hand, the binding of MUC1 to adhesion molecules expressed on endothelial cells, such as selectin-like molecules might eventually contribute to the hematogenous metastatic spread.47 Such mechanisms explained the clinical findings that MUC1 overexpression leads to tumor metastasis and poor prognosis in human epithelial cancers.
Subgroup analyses revealed that worse OS with high MUC1 could be found in many cancers, such as gastric cancer, colorectal cancer, cholangiocarcinoma, and NSCLC regardless of different analysis strategies and antibodies. Compared with Caucasian people, increased MUC1 expression was more significantly associated with poor OS in Asian population (HRFEM = 2.28, 95%CIFEM: 1.90–2.72, PFEM = 0.683; HRREM = 2.27,95% CIREM: 1.50–3.46, PREM = 0.638). Further, considering different cut-off values, follow-up time, and antibodies, meta-regression was performed to investigate the source of heterogeneity. However, none of the variables listed above contributed to the heterogeneity in our meta-analysis. Additionally, when the clinicopathological features were considered, MUC1 overexpression was more likely to be found in colorectal cancer patients with an advanced TNM stage and in gastric cancer patients with positive lymph node metastasis and intestinal-type classification. To date, most immunotherapy approaches targeting MUC1 have been administered to >1200 patients in clinical trials. As a notable example, synthetic vaccine ImMucin successfully completed a Phase I/II clinical trial in multiple myeloma patients.48 Recently, Stimuvax has been developed and has completed a Phase III trial in patients with IIIA/B NSCLC. However, few anti-MUC1 immunotherapy trials were explored in gastrointestinal cancers during 1996 to 2012. Our results suggest that anti-MUC1 immunotherapy might be preferentially carried out on patients with gastrointestinal cancers in future clinical trials.
Although our results are promising, there are several limitations of this study need to be carefully taken into account. First, as a novel prognostic marker in malignancies, MUC1 just loomed in recent years and nearly half of the sample size was relatively small. Second, all of the selected studies measured MUC1 expression by IHC, the distinct antibodies used, the protocol of staining, the different cut-off values, and the method of scoring might account for the high variability in the frequencies reported by different authors. In addition, although triple subsets of MUC1 threshold values revealed the similar results, a baseline referring to MUC1 overexpression should be set up. Third, few studies explored the association of patient prognosis and circulating MUC1 (also known as CA15–3) expression, which might be more acceptable than tissue markers because they can be assayed before surgery and be monitored throughout the life. Finally, due to the lack of appropriate data, most studies included in this meta-analysis focused on gastrointestinal neoplasms and NSCLC. In the future, studies with more types of cancers and larger sample size are needed to present more convincible results of the clinical relevance and precise molecular explanation for the abnormal expression of MUC1.
In conclusion, the current evidence first shows that an elevated MUC1 is a negative predictor for survival in epithelial-originated cancers, especially in NSCLC and gastrointestinal tumors.
Footnotes
Abbreviations: BC = breast cancer, CC = cholangiocarcinoma, CI = confidence interval, CRC = colorectal cancer, EMT = epithelial to mesenchymal transition, FEM = fixed effect model, GBC = gallbladder carcinoma, GC = gastric carcinoma, HR = hazard ratio, MAB = monoclonal antibody, MOOSE = Meta-Analysis of Observational Studies in Epidemiology group, MUC1 = mucin 1, NOS = Newcastle–Ottawa Scale, NSCLC = nonsmall cell lung cancer, OR = odds ratio, OS = overall survival, OSC = coral squamous cell carcinoma, PC = pancreatic cancer, REM = random effect model, SGMEC = salivary gland mucoepidermoid carcinoma, UCC = uterine cervical carcinoma.
Funding: This study was supported by the National Science and Technology Support Program of China (2013BAI04B01).
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: Cancer J Clin 2015; 65:5–29. [DOI] [PubMed] [Google Scholar]
- 2.Paul D, Kumar A, Gajbhiye A, et al. Mass spectrometry-based proteomics in molecular diagnostics: discovery of cancer biomarkers using tissue culture. Biomed Res Int 2013; 2013:783131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang JF, Zhao HL, Phillips J, et al. The epithelial mucin, MUC1, is expressed on resting T lymphocytes and can function as a negative regulator of T cell activation. Cell Immunol 2000; 201:83–88. [DOI] [PubMed] [Google Scholar]
- 4.Lau SK, Weiss LM, Chu PG. Differential expression of MUC1, MUC2, and MUC5AC in carcinomas of various sites: an immunohistochemical study. Am J Clin Pathol 2004; 122:61–69. [DOI] [PubMed] [Google Scholar]
- 5.Wesseling J, van der Valk SW, Vos HL, et al. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol 1995; 129:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schroeder JA, Masri AA, Adriance MC, et al. MUC1 overexpression results in mammary gland tumorigenesis and prolonged alveolar differentiation. Oncogene 2004; 23:5739–5747. [DOI] [PubMed] [Google Scholar]
- 7.Kimura T, Finn OJ. MUC1 immunotherapy is here to stay. Expert Opin Biol Ther 2013; 13:35–49. [DOI] [PubMed] [Google Scholar]
- 8.Ilhan O, Han U, Onal B, et al. Prognostic significance of MUC1, MUC2 and MUC5AC expressions in gastric carcinoma. Turk J Gastroenterol 2010; 21:345–352. [DOI] [PubMed] [Google Scholar]
- 9.Baldus SE, Monig SP, Huxel S, et al. MUC1 and nuclear beta-catenin are coexpressed at the invasion front of colorectal carcinomas and are both correlated with tumor prognosis. Clin Cancer Res 2004; 10:2790–2796. [DOI] [PubMed] [Google Scholar]
- 10.Winter JM, Tang LH, Klimstra DS, et al. A novel survival-based tissue microarray of pancreatic cancer validates MUC1 and mesothelin as biomarkers. PLoS One 2012; 7:e40157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Vegt B, de Roos MA, Peterse JL, et al. The expression pattern of MUC1 (EMA) is related to tumour characteristics and clinical outcome of invasive ductal breast carcinoma. Histopathology 2007; 51:322–335. [DOI] [PubMed] [Google Scholar]
- 12.Sivridis E, Giatromanolaki A, Koukourakis MI, et al. Patterns of episialin/MUC1 expression in endometrial carcinomas and prognostic relevance. Histopathology 2002; 40:92–100. [DOI] [PubMed] [Google Scholar]
- 13.Hirasawa Y, Kohno N, Yokoyama A, et al. Natural autoantibody to MUC1 is a prognostic indicator for non-small cell lung cancer. Am J Respir Crit Care Med 2000; 161 (2 Pt 1):589–594. [DOI] [PubMed] [Google Scholar]
- 14.Abd Elazeez TA, El-Balshy Ael L, Khalil MM, et al. Prognostic significance of P27 (Kip 1) and MUC1 in papillary transitional cell carcinoma of the urinary bladder. Urol Ann 2011; 3:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25:603–605. [DOI] [PubMed] [Google Scholar]
- 16.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 19.Xie S, Wang K, Xu H, et al. PRISMA-extracapsular dissection versus superficial parotidectomy in treatment of benign parotid tumors: evidence from 3194 patients. Medicine 2015; 94:e1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGuckin MA, Walsh MD, Hohn BG, et al. Prognostic significance of MUC1 epithelial mucin expression in breast cancer. Human Pathol 1995; 26:432–439. [DOI] [PubMed] [Google Scholar]
- 21.Guddo F, Giatromanolaki A, Koukourakis MI, et al. MUC1 (episialin) expression in non-small cell lung cancer is independent of EGFR and c-erbB-2 expression and correlates with poor survival in node positive patients. J Clin Pathol 1998; 51:667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baldus SE, Zirbes TK, Engel S, et al. Correlation of the immunohistochemical reactivity of mucin peptide cores MUC1 and MUC2 with the histopathological subtype and prognosis of gastric carcinomas. Int J Cancer 1998; 79:133–138. [DOI] [PubMed] [Google Scholar]
- 23.Utsunomiya T, Yonezawa S, Sakamoto H, et al. Expression of MUC1 and MUC2 mucins in gastric carcinomas: its relationship with the prognosis of the patients. Clin Cancer Res 1998; 4:2605–2614. [PubMed] [Google Scholar]
- 24.Higashi M, Yonezawa S, Ho JJ, et al. Expression of MUC1 and MUC2 mucin antigens in intrahepatic bile duct tumors: its relationship with a new morphological classification of cholangiocarcinoma. Hepatology 1999; 30:1347–1355. [DOI] [PubMed] [Google Scholar]
- 25.Matsumura N, Yamamoto M, Aruga A, et al. Correlation between expression of MUC1 core protein and outcome after surgery in mass-forming intrahepatic cholangiocarcinoma. Cancer 2002; 94:1770–1776. [DOI] [PubMed] [Google Scholar]
- 26.Lee HS, Lee HK, Kim HS, et al. MUC1, MUC2, MUC5AC, and MUC6 expressions in gastric carcinomas: their roles as prognostic indicators. Cancer 2001; 92:1427–1434. [DOI] [PubMed] [Google Scholar]
- 27.Baldus SE, Monig SP, Hanisch FG, et al. Comparative evaluation of the prognostic value of MUC1, MUC2, sialyl-Lewis(a) and sialyl-Lewis(x) antigens in colorectal adenocarcinoma. Histopathology 2002; 40:440–449. [DOI] [PubMed] [Google Scholar]
- 28.Akyurek N, Akyol G, Dursun A, et al. Expression of MUC1 and MUC2 mucins in gastric carcinomas: their relationship with clinicopathologic parameters and prognosis. Pathol Res Pract 2002; 198:665–674. [DOI] [PubMed] [Google Scholar]
- 29.Wang JY, Chang CT, Hsieh JS, et al. Role of MUC1 and MUC5AC expressions as prognostic indicators in gastric carcinomas. J Surg Oncol 2003; 83:253–260. [DOI] [PubMed] [Google Scholar]
- 30.Nagai S, Takenaka K, Sonobe M, et al. A novel classification of MUC1 expression is correlated with tumor differentiation and postoperative prognosis in non-small cell lung cancer. J Thorac Oncol 2006; 1:46–51. [PubMed] [Google Scholar]
- 31.Duncan TJ, Watson NF, Al-Attar AH, et al. The role of MUC1 and MUC3 in the biology and prognosis of colorectal cancer. World J Surg Oncol 2007; 5:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woenckhaus M, Merk J, Stoehr R, et al. Prognostic value of FHIT, CTNNB1, and MUC1 expression in non-small cell lung cancer. Human Pathol 2008; 39:126–136. [DOI] [PubMed] [Google Scholar]
- 33.Togami S, Nomoto M, Higashi M, et al. Expression of mucin antigens (MUC1 and MUC16) as a prognostic factor for mucinous adenocarcinoma of the uterine cervix. J Obstet Gynaecol Res 2010; 36:588–597. [DOI] [PubMed] [Google Scholar]
- 34.Park SY, Roh SJ, Kim YN, et al. Expression of MUC1, MUC2, MUC5AC and MUC6 in cholangiocarcinoma: prognostic impact. Oncol Rep 2009; 22:649–657. [DOI] [PubMed] [Google Scholar]
- 35.Ando H, Aihara R, Ohno T, et al. Prognostic significance of the expression of MUC1 and collagen type IV in advanced gastric carcinoma. Br J Surg 2009; 96:901–909. [DOI] [PubMed] [Google Scholar]
- 36.Westgaard A, Schjolberg AR, Cvancarova M, et al. Differentiation markers in pancreatic head adenocarcinomas: MUC1 and MUC4 expression indicates poor prognosis in pancreatobiliary differentiated tumours. Histopathology 2009; 54:337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Retterspitz MF, Monig SP, Schreckenberg S, et al. Expression of {beta}-catenin, MUC1 and c-met in diffuse-type gastric carcinomas: correlations with tumour progression and prognosis. Anticancer Res 2010; 30:4635–4641. [PubMed] [Google Scholar]
- 38.Situ D, Wang J, Ma Y, et al. Expression and prognostic relevance of MUC1 in stage IB non-small cell lung cancer. Medical Oncol (Northwood, London, England) 2011; 28 Suppl 1:S596–S604. [DOI] [PubMed] [Google Scholar]
- 39.Kim SM, Oh SJ, Hur B. Expression of MUC1 and MUC4 in gallbladder adenocarcinoma. Korean J Pathol 2012; 46:429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamada T, Nomura M, Kamikawa Y, et al. DF3 epitope expression on MUC1 mucin is associated with tumor aggressiveness, subsequent lymph node metastasis, and poor prognosis in patients with oral squamous cell carcinoma. Cancer 2012; 118:5251–5264. [DOI] [PubMed] [Google Scholar]
- 41.Khanh do T, Mekata E, Mukaisho K, et al. Transmembrane mucin MUC1 overexpression and its association with CD10(+) myeloid cells, transforming growth factor-beta1 expression, and tumor budding grade in colorectal cancer. Cancer Sci 2013; 104:958–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu S, Ruan M, Li S, et al. Increased expression of MUC1 predicts poor survival in salivary gland mucoepidermoid carcinoma. J Craniomaxillofac Surg 2014; 42:1891–1896. [DOI] [PubMed] [Google Scholar]
- 43.Lacunza E, Baudis M, Colussi AG, et al. MUC1 oncogene amplification correlates with protein overexpression in invasive breast carcinoma cells. Cancer Genet Cytogenet 2010; 201:102–110. [DOI] [PubMed] [Google Scholar]
- 44.Lagow EL, Carson DD. Synergistic stimulation of MUC1 expression in normal breast epithelia and breast cancer cells by interferon-gamma and tumor necrosis factor-alpha. J Cell Biochem 2002; 86:759–772. [DOI] [PubMed] [Google Scholar]
- 45.Rajabi H, Jin C, Ahmad R, et al. Mucin 1 oncoprotein expression is suppressed by the mir-125b oncomir. Genes Cancer 2010; 1:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roy LD, Sahraei M, Subramani DB, et al. MUC1 enhances invasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition. Oncogene 2011; 30:1449–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gendler SJ. MUC1, the renaissance molecule. J Mammary Gland Biol Neoplasia 2001; 6:339–353. [DOI] [PubMed] [Google Scholar]
- 48.Kovjazin R, Volovitz I, Kundel Y, et al. ImMucin: a novel therapeutic vaccine with promiscuous MHC binding for the treatment of MUC1-expressing tumors. Vaccine 2011; 29:4676–4686. [DOI] [PubMed] [Google Scholar]


