Supplemental Digital Content is available in the text
Abstract
This study aimed to explore the clinical significance of breast tumor tissue stiffness based on ultrasound elastographic evaluation in clinical breast cancer.
Tumor tissue stiffness is mainly regulated by interactions among tumor cells, stromal cells, and extracellular matrix and was recently regarded as a representative feature of tumor microenvironment. Basic research has already revealed that the tumor stiffness can lead to tumor progression; however, little is known about its clinical significance because thus far, no useful modality is available in the clinical setting.
We investigated the tumor stiffness by strain elastography in 503 consecutive patients with invasive breast cancer. Correlations between stiffness and clinicopathological factors, including tumor size, lymph node involvement, tumor subtypes, and stromal-related genes’ expressions in primary breast tumor, were statistically examined.
We identified that clinical tumor stiffness significantly correlated with lymph node involvement and invasive tumor size but not with hormonal receptor expressions, human epidermal growth factor receptor type 2 status, and ki67 labeling index by analyses of both categorical and continuous variables of stiffness. On multivariate analyses, axillary lymph node metastasis was an independent factor that influenced the stiffness of primary breast tumor. In the gene expression analyses, relatively hard tumors had a significantly high gene expression of lysyl oxidase compared with soft tumors.
Our study showed a close relationship between primary tumor stiffness by elastographic evaluation and lymph node involvement in clinical breast cancer. Further investigations on tumor-related tissue stiffness are required.
INTRODUCTION
Recent technological innovations, such as novel alterations by next-generation sequencing, can remarkably advance cancer research. These researches have steadily revealed the molecular mechanism of cancer hallmarks1; however, many challenges still exist, such as tumor heterogeneity or cancer stem cells property.2–5 Nowadays, there has been an expansion in cancer research from cancer cell-centric approach to adjacent surrounding trademarks known as tumor microenvironment.6 Based on recent basic research, this property mainly consists of the interactions among cancer cells, stroma, and stromal cells, including cancer-associated fibroblasts and immune cells, and significantly contributes to cancer initiation and progression.7
For instance, one of the characteristics of tumor microenvironment is the presence of tumor-infiltrating lymphocytes, and this feature has become a recent topic of debate because of its potential clinical significance of predicting the efficacy of systemic therapy and its prognostic value in several cancer.8,9 Along with the emerging significance of the tumor environment in addition to cancer cells alone, novel treatment strategies, including anti-angiogenesis therapy10 and immune checkpoint inhibitors,11 have been applied in the clinical setting.
Another attractive feature of tumor microenvironment is tumor-associated tissue stiffness. Basic research has already indicated that tumor stiffness can influence tumor progression by involving the extracellular matrix (ECM) and β1-integrin/phosphoinositide 3 kinase (PI3K) pathway.12,13 Tissue stiffness is clinically known as an oncogenic risk factor correlated with dense breast and liver cirrhosis; in addition, tumor stiffness is one of the characteristics that clinicians look for during palpation of several cancers. However, thus far, there have been few useful quantitative modalities, particularly in clinical practice and reverse translational research.14
More recently, ultrasound elastography has been applied for several clinical situations, such as measuring the level of fibrosis in liver cirrhosis and differentiating certain malignant tumors, including breast cancer. Elastography can objectively and noninvasively evaluate the stiffness of a target region during conventional sonographic examinations.15,16 There is an urgent need to identify useful factors and biomarkers to promote optimal individualized treatment.
Therefore, the aim of this study was to explore the significance of clinical tumor stiffness by elastographic evaluation in patients with invasive breast cancer by investigating the correlations between clinical stiffness and clinicopathological factors. In addition, analysis of stiffness-related gene expression was also performed with the aim of correlating basic and clinical researches.12,13,17
METHODS
Patients
This was a retrospective study on 503 consecutive women with invasive breast cancer who underwent evaluation of clinical tumor stiffness and treatments at Kumamoto University Hospital, Japan between February 2007 and January 2014. Informed consent was obtained from all patients, and this study followed the guidelines of the ethics committee of Kumamoto University Graduate School of Medical Sciences, which also granted the approval for the gene expression analysis (GEA) in the study.
Measurement and Evaluation of Clinical Tumor Stiffness
In this study, we measured the macro stiffness of breast cancer tissue with ultrasound strain elastography (EUB-8500; Hitachi-Aloka Medical, Tokyo, Japan) concurrent with conventional ultrasound examination before any treatment for breast cancer. Detailed evaluation methods are described in our previous study and in the guidelines on ultrasound elastography.15,18 To evaluate tumor stiffness, we used Strain Ratio, a semi-quantitative method to assess how many times stiffer a target tumor was compared with a control lesion in this study; in detail, we used Fat Lesion Ratio (FLR) that using subcutaneous fat as control. FLR has been used commonly for differentiation of breast disease because subcutaneous fat had the most stable stiffness in breast lesions.19,20 If patient has multiple tumor lesions, we give priority to the stiffer evaluation over softer evaluation.
Assessment of Clinicopathological Features in Breast Cancer
Assessment of breast cancer biomarkers, including estrogen receptor (ER), progesterone receptor (PgR), human epidermal growth factor receptor type 2 (HER2), and Ki67 labeling index, was prospectively performed for each patient to guide treatment strategy.21 Immunohistochemical staining was done as previously described.22 Briefly, ER and PgR status were considered positive when there was ≥1% of nuclear staining. HER2 positivity was indicated by 3+ immunohistochemical staining or fluorescence in situ hybridization with a threshold ratio of >2.2.23 Ki67 labeling index was determined by counting at least 500 tumor cells in hot spots. Masson's trichrome staining was performed according to the manufacture's recommended protocol (Sigma–Aldrich, St Lois, MO).
Tumor size was generally assessed based on the pathologically invasive area in the surgical specimen; in patients who received any neoadjuvant therapy, tumor size was assessed by pretreatment magnetic resonance imaging or sonography. Evaluation of axillary lymph node metastasis was pathologically performed and classified according to pTNM staging system (UICC, Seventh Edition, 2009). The pathologists in Kumamoto University Hospital assessed Nuclear Grade based on Atypia score and Mitotic index.
GEA of Breast Cancer Tissue
Of 503 cases, 164 breast cancer tissues were available in this study period underwent pretreatment evaluation of clinical tumor stiffness and fresh frozen tissue sampling for GEA. RNA extraction and quantitative real-time reverse-transcription polymerase chain reaction (qRT-PCR) were performed as previously described.24 The main primers used for qRT-PCR analysis were as follows: lysyl oxidase (LOX) forward 5′-TATACATAAGGCAGCCGTGAA-3′, LOX reverse 5′-GAGACAGTTGTGGTTTGGG-3′; hypoxia-inducible factor 1-alpha (HIF1A) forward 5′-CGTTCCTTCGATCAGTTGTC-3′, HIF1A reverse 5′-TCAGTGGTGGCAGTGGTAGT-3′; secreted phosphoprotein 1 (SPP1) forward 5′-AGATGCAGCACCGAGGCT-3′, SPP1 reverse 5′-CTTTCTTTTTGGCGACCG-3′; and cylindromatosis (CYLD) forward 5′-TCAGGCTTATGGAGCCAAGAA-3′, CYLD reverse 5′-ACTTCCCTTCGGTACTTTAAGGA-3′. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control.
Statistical Analysis
The significance of differences among categorical and continuous variables was evaluated using univariate logistic regression model, Chi-squared and nonparametric Wilcoxon/Kruskal–Wallis tests, respectively. Cauchy False Discovery Rate (FDR)-adjusted P value was also calculated in GEA. Univariate and multivariate analyses of factors influencing tumor stiffness, lymph node involvement and tumor size were performed with a logistic regression model. Stepwise regression method (P < 0.25, forward and backward) was used to build the final multivariate model. P value < 0.05 was considered to be statistically significant. JMP software version 12.0.1 for MAC (SAS Institute Japan, Tokyo, Japan) was used for these statistical analyses.
RESULTS
Correlations Between Clinical Tumor Stiffness and Clinicopathological Factors in Patients With Breast Cancer
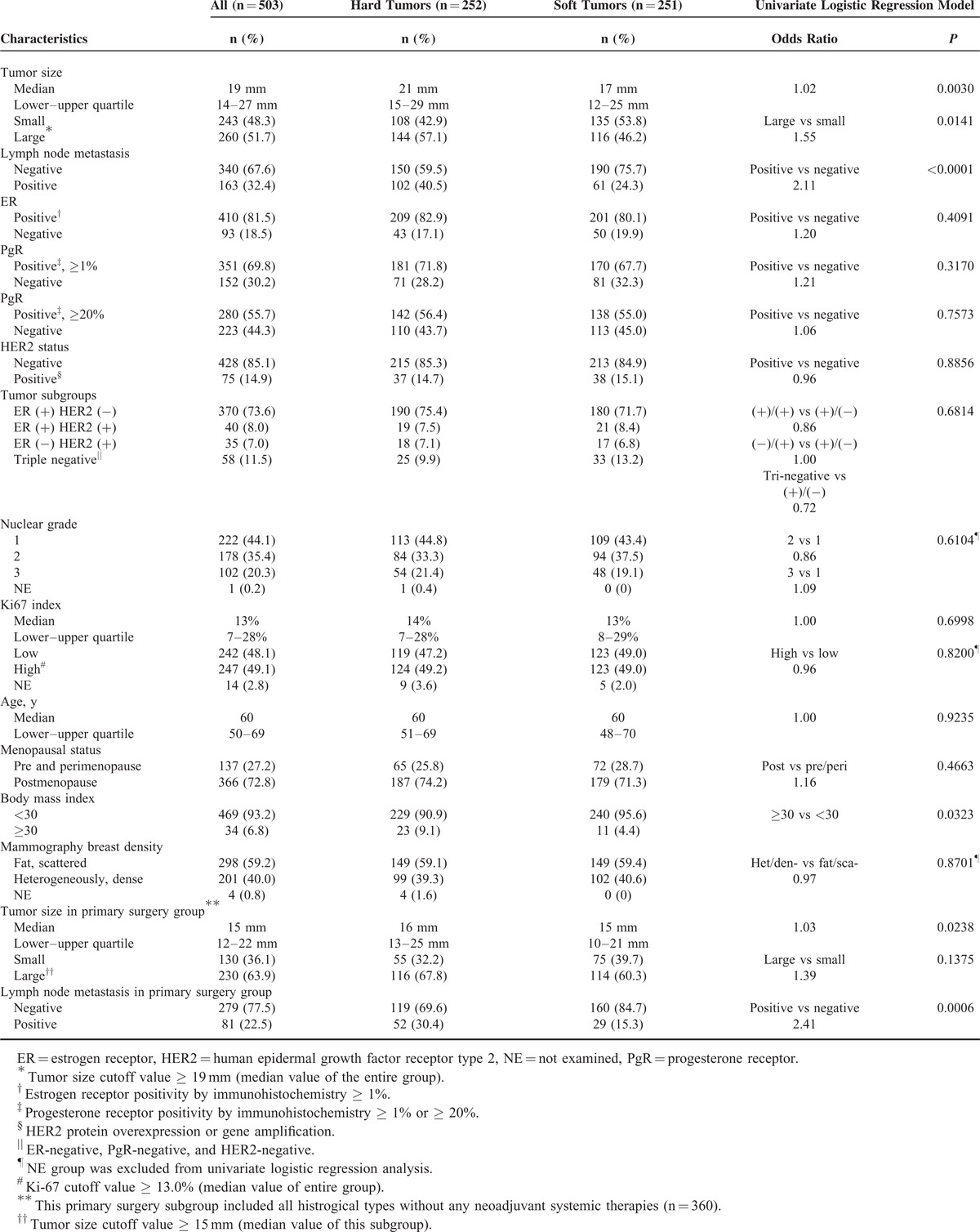
The baseline characteristics of 503 patients with invasive breast cancer are shown in Table 1. To clarify the significance of clinical tumor stiffness in breast cancer, we categorized tumors into 2 groups according to the elastographic evaluation with a cutoff value of 8.23 as median Strain Ratio (interquartile range [IQR] 3.92–23.36]; Table 1). Compared with relatively soft tumors, relatively hard tumors (Supplemental Figure 1) had significant correlations with the frequency of axillary lymph node metastasis (hard tumors: 40.5% vs soft tumors: 24.3%, P < 0.0001 in univariate logistic regression model), large tumor size (hard tumors: 57.1% vs soft tumors: 46.2% of larger tumor occupancy, P = 0.0141 in univariate logistic regression model), and high body mass index (BMI) index of patients (hard tumors: 9.1% vs soft tumors: 4.4% of BMI ≥ 30, P = 0.0323 in univariate logistic regression model). There were no clear correlations between tumor stiffness and conventional biomarkers such as ER (P = 0.4091), PgR (P = 0.3170), HER2 status (P = 0.8856), and Ki67 labeling index (P = 0.8200 in univariate logistic regression model).
TABLE 1.
Baseline Characteristics of Patients With Breast Cancer According to Tumor Stiffness
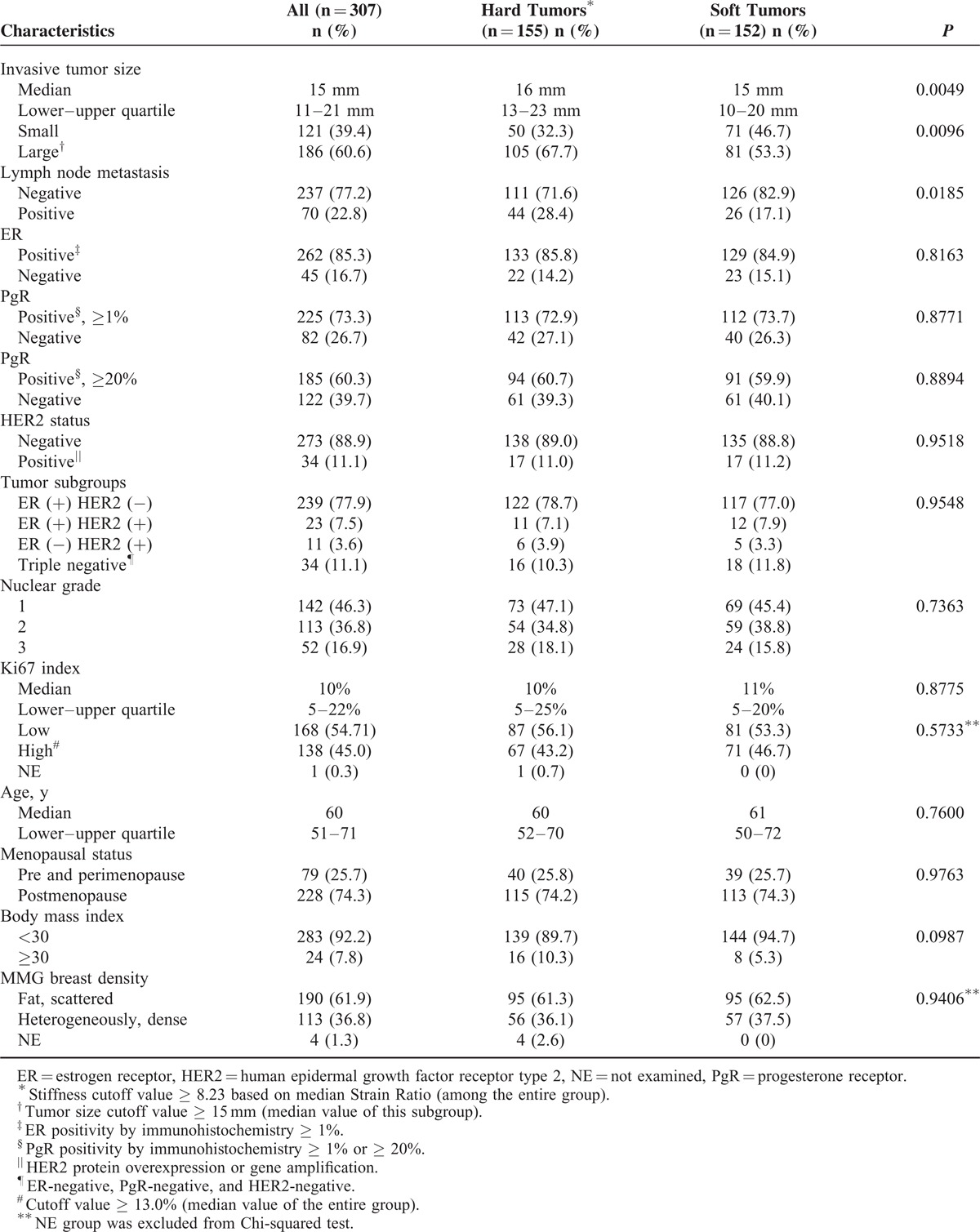
When we analyzed Strain Ratio as a continuous variable, there were similar results on correlations between tumor stiffness and lymph node metastasis (median Strain Ratio, 13.46 in metastasis-positive vs 7.00 in metastasis-negative cases, P = 0.0001), invasive tumor size (median Strain Ratio, 11.98 in large tumors vs 7.19 in small tumors, P = 0.0020), and high BMI index (median Strain Ratio, 22.41 in higher BMI vs 7.97 in other cases, P = 0.0096; Table 2). Even in the subgroup of 307 patients with histologically invasive ductal carcinoma (IDC) who underwent primary surgery (Table 3), there were also significant correlations between clinical tumor stiffness and invasive tumor size (P = 0.0096 in nonparametric Wilcoxon test) or axillary lymph node metastasis (P = 0.0185 in Chi-squared test).
TABLE 2.
Correlations Between Strain Ratio as a Continuous Variable and Clinicopathological Factors in Breast Cancer
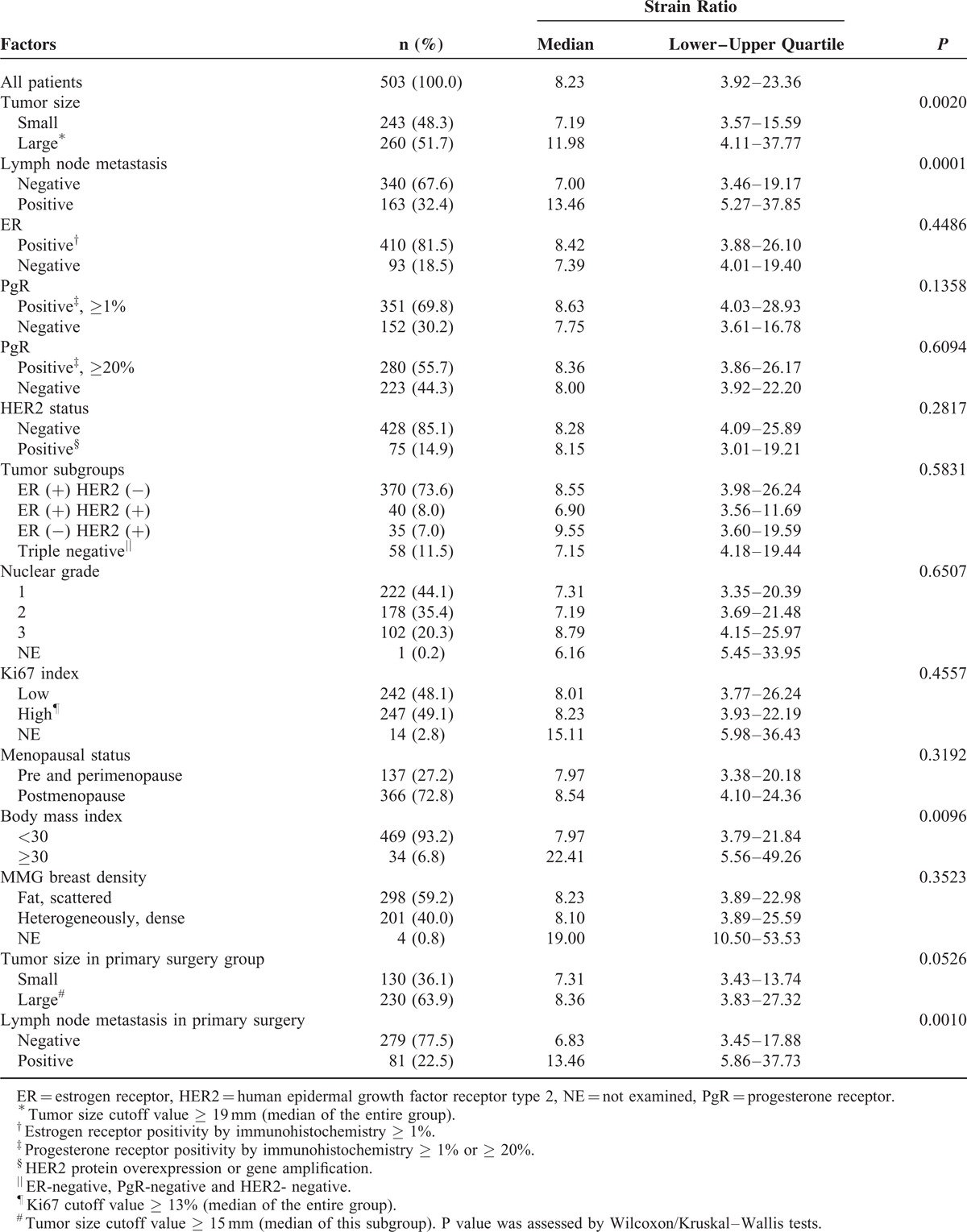
TABLE 3.
Restriction Analysis in Patients With Histologically Invasive Ductal Carcinoma Who Received Primary Surgery
Collectively, by strain elastography, relatively hard breast cancer was strongly correlated with worse clinical features, such as axillary lymph node involvement, large invasive tumor size, and obesity.
Multivariate Analysis for Factors Influencing Clinical Tumor Stiffness in Patients With Breast Cancer
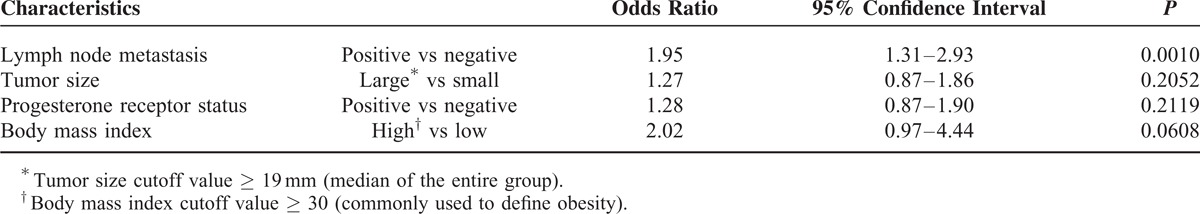
Among representative characteristics of clinical breast cancer, axillary lymph node metastasis was an independent factor [odds ratio (OR) 1.95, 95% confidence interval (CI) 1.31–2.93, P = 0.0010], but not ER, PgR, HER2, or Ki67 index (Table 4). In the subgroup of patients with histologically IDC who underwent primary surgery, independent factors influencing tumor stiffness were lymph node metastasis (OR: 1.80, 95% CI: 1.02–3.24, P = 0.0426) and tumor size (OR: 1.81, 95% CI: 1.10–2.99, P = 0.0184; Supplemental Table 1).
TABLE 4.
Multivariate Analysis of Factors Influencing Clinical Tumor Stiffness in Breast Cancer (n = 503)
In contrast, multivariate analysis of factors influencing axillary lymph node involvement indicated clinical tumor stiffness (OR: 2.07, 95% CI: 1.37–3.15, P = 0.0005), tumor size (OR: 3.51, 95% CI: 2.30–5.42, P ≤ 0.0001), and Ki67 labeling index (OR: 2.17, 95% CI: 1.43–3.30, P = 0.0003) as independent predictors (Supplemental Table 2). However, when we performed similar multivariate analysis of factors influencing tumor size, tumor stiffness was not significant (OR: 1.30, 95% CI: 0.88–1.90, P = 0.1845).
Focusing on nodal staging, there were significant differences in percentages of pathological lymph node stage (pN) according to clinical tumor stiffness (pN0: 150/503, 29.8%; pN1: 75/503, 14.9%; pN2: 19/503, 3.8%; and pN3: 8/503, 1.6% in hard tumors vs pN0: 190/503, 37.8%; pN1: 45/503, 9.0%; pN2: 10/503, 2.0%; and pN3: 6/503, 1.2% in soft tumors; P = 0.0016; Supplemental Figure 2A). In addition, Strain Ratio as a continuous variable was also significant with respect to each pN stage (median value of 7.0 in pN0, 13.18 in pN1, 13.89 in pN2, and 16.43 in pN3; P = 0.0015; Supplemental Figure 2B).
Correlations Between Clinical Tumor Stiffness and Stroma-Related Genes’ Expressions
We examined the correlations between clinical tumor stiffness and stroma-related gene expressions, including LOX, HIF1A, SPP1, or Osteopontin and CYLD, that were extracted from macro tumor tissues of 164 patients with breast cancer. When we divided these patients into 2 groups based on a cutoff median Strain Ratio of 8.23 (IQR 4.00–34.62) in this subgroup, no significant difference in baseline characteristics was observed except for BMI (Supplemental Table 3).
Compared with soft tumors, hard tumors had higher mRNA expression of LOX (median 295.7, IQR 152.6–734.2 vs median 173.8, IQR 76.3–453.9; P = 0.0072 in nonparametric Wilcoxon test, FDR-adjusted P = 0.0279; Figure 1A), and a tendency for higher expression of HIF1A (median 6.94, IQR 4.67–10.88 vs median 5.97, IQR 3.75–9.16; P = 0.0839 in nonparametric Wilcoxon test, FDR-adjusted P = 0.2444; Figure 1B). There was no obvious correlation between tumor stiffness and SPP1 mRNA expression (median 83.0, IQR 48.0–173.0 in hard tumors vs median 88.0, IQR 33.0–197.0 in soft tumors, P = 0.7124 in nonparametric Wilcoxon test, FDR-adjusted P = 0.4681; Figure 1C) or CYLD mRNA expression (median 1.27, IQR 0.74–2.38 in hard tumors vs median 1.42, IQR 0.73–2.12 in soft tumors, P = 0.7310 in nonparametric Wilcoxon test, FDR-adjusted P = 0.5482; Figure 1D). Multivariate logistic regression model of factors influencing clinical tumor stiffness in the GEA group indicated LOX mRNA expression (OR: 3.48, 95% CI: 1.74–7.24, P = 0.0004), and body mass index (OR: 2.29, 95% CI: 1.09–4.99, P = 0.0290) as independent factors (Supplemental Table 4).
FIGURE 1.
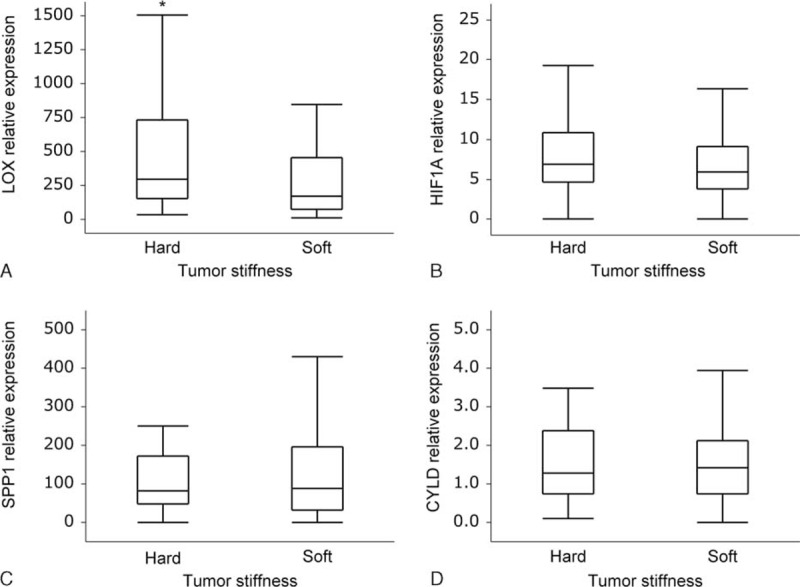
mRNA expressions of stroma-related genes according to clinical tumor stiffness in patients with breast cancer. (A) Relative expression of LOX was significantly high in hard tumors (median 295.7, IQR 152.6–734.2) compared with that in soft tumors (median 173.8, IQR 76.3–453.9); ∗P = 0.0072. (B) Relative expression of HIF1A tended to be high in hard tumors (median 6.94, IQR 4.67–10.88) compared with that in soft tumors (median 5.97, IQR 3.75–9.16); P = 0.0839. (C) There was no significant difference in SPP1-relative expression between hard (median 83.0, IQR 48.0–173.0) and soft tumors (median 88.0, IQR 33.0–197.0); P = 0.7124. (D) There was no significant difference in CYLD-relative expression between hard (median 1.27, IQR 0.74–2.38) and soft tumors (median 1.42, IQR 0.73–2.12); P = 0.7310. P values were evaluated using nonparametric Wilcoxon test. CYLD = cylindromatosis, HIF1A = hypoxia-inducible factor 1-alpha, IQR = interquartile range, LOX = lysyl oxidase, SPP1 = secreted phosphoprotein 1.
DISCUSSION
Tissue stiffness is a significant malignant property of various tumors, including breast cancer, which can force tumor development in tumor cell-autonomous and noncell-autonomous manners. Basic research has revealed these molecular mechanisms, and in addition, an epidemiological study has suggested a causational link between relative risk of breast cancer and dense breast on mammography. However, there has been little evidence on clinical tumor stiffness and tumor progression in clinical or translational research.
The most important finding from this study was that tumor stiffness by elastographic evaluation may have clinical significance for certain patients with breast cancer. Clinical tumor stiffness of a primary breast cancer significantly correlated with axillary lymph node involvement; in contrast, it did not correlate with current potent biomarkers, such as ER, PgR, and HER2 status. Although there is little causal evidence from this study, our current data may support basic research findings that local tissue stiffness of a tumor enhanced tumor cell metastasis.25
Similar to the present study, Evans et al26 recently reported that tumor stiffness based on shear-wave elastography was an independent predictor of lymph node metastasis in 396 breast cancer patients. In contrast, Youk et al27 reported that the independent factors that influenced breast cancer stiffness by shear-wave elastography in 152 patients were lymphovascular invasion in the primary tumor, palpable abnormality, and histological grade but not axillary lymph node metastasis, ER, PgR, and Ki67 status. Although different from our study in terms of stiffness measurement15 or analytical direction on multivariate analysis, there was a similar finding that tumor stiffness by evaluation of ultrasound elastography may closely correlate with lymphatic metastasis in clinical breast cancer. In addition, our previous investigation showed a potential link between clinical tumor stiffness by strain elastography and efficacy of neoadjuvant chemotherapy in 55 patients with breast cancer.18 Beyond the significance on differential diagnosis, further researches focusing on clinical tumor stiffness should be conducted.
The limitation of this study was the reproducibility of stiffness evaluation by ultrasound elastography, which continues to be an important task as with any imaging system.15 In the present study, we used Strain Ratio both as categorical variable, using median value as cutoff, and as continuous variable to reduce the influence of measuring error. In the future, it may be possible to define a more appropriate and practical cutoff by further standardization of ultrasound elastography system.
In terms of biological mechanism, the stiffness of tumor tissue is regulated by several key factors, such as tumor cells, stromal cell, stroma, and hydrostatic pressure.28 At least in breast cancer, stromal property, particularly ECM, seems to be more important element in the tumor hardness.29 Consecutive researches by Weaver et al have revealed that the structure, orientation, and quantity of ECM collagens play a key role in regulating tumor stiffening and tumor development via integrin, focal adhesion kinase, phosphatase and tensin homolog, and PI3K on mammary epithelial cells.25,30–32 LOX derived from both tumor cells and stromal cells plays critical roles in tissue stiffening and promotes breast cancer metastasis by working as an extracellular copper enzyme.25,31,33
We therefore examined the stroma-related gene expressions on available frozen macro tissues as well as the correlations between clinical tumor stiffness and clinicopathological factors. In particular, we focused on the gene expressions of collagen cross-linking enzyme LOX, hypoxia factor HIF1α correlated tissue stiffening and stimulating LOX expression, fibrosis and chemotherapy resistance factor SPP1, and novel NF-κB regulator CYLD. There was a strong correlation between tumor stiffness by ultrasound elastography and LOX mRNA expression. Since basic research has revealed that close-interaction between LOX and hypoxia can induce tumor-associated stiffness and premetastatic niche,31,34 our translational research may support the biological mechanism among LOX expression, tumor-associated tissue stiffness, and tumor cell metastasis. Tumor stiffness did not correlate with other fibrosis-related gene expressions, such as SPP135 and CYLD.36 Since little is known about correlation of gene expressions with tumor stiffness by elastography, further investigation is required to estimate the clinical utility of this evaluation system.
Moreover, there was also a correlation between tumor stiffness and obesity in this analysis. It remains unclear whether the method of measurement or biological mechanism affected this correlation because FLR assay is a semi-quantitative method as previously described.20,37 Recent research showed that the progression of obesity-related breast cancer was closely associated with chronic inflammation, which often induced tissue hardening.38 Further research is required to clarify these unresolved questions.
CONCLUSIONS
New evidence will hopefully emerge from this study on tumor progressions and tumor-associated stiffness in its microenvironment. In the future, clinical evaluation of stiffness by ultrasound elastography may become a useful imaging biomarker because stiffness has a potential to be regarded as a clinical phenotype of various tumor interactions. In clinical breast cancer, the status of axillary lymph node metastasis remains to be a strong prognostic or treatment decision-making factor; however, the choice of treatment among sentinel node biopsy, local radiotherapy, and omission of dissection surgery is currently a controversial subject.21 As further validation of the close relationship between breast cancer and lymph node metastasis progresses, evaluation of clinical tumor stiffness may help patients receive appropriate treatments.
Supplementary Material
Acknowledgments
We are grateful to M. Komi and K. Shimizu for their excellent technical support, to M. Suematsu and M. Tanabe for their great management of clinical data and to the other members of Breast and Endocrine Surgery at Graduate School of Medical Sciences, Kumamoto University for their kind supports.
Footnotes
Abbreviations: BMI = body mass index, CYLD = cylindromatosis, ECM = extracellular matrix, ER = estrogen receptor, FLR = fat resin ratio, GEA = gene expression analysis, HER2 = human epidermal growth factor receptor type 2, HIF1A = hypoxia inducible factor 1 alpha, IDC = invasive ductal carcinoma, IQR = interquartile range, LOX = lysyl oxidase, PgR = progesterone receptor, PI3K = phosphoinositide 3 kinase, SPP1 = secreted phosphoprotein 1.
This work was supported by JSPS KAKENHI Grant Number 15K21239.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646–674. [DOI] [PubMed] [Google Scholar]
- 2.Woll PS, Kjallquist U, Chowdhury O, et al. Myelodysplastic syndromes are propagated by rare and distinct human cancer stem cells in vivo. Cancer Cell 2014; 25:794–808. [DOI] [PubMed] [Google Scholar]
- 3.Kai K, Arima Y, Kamiya T, et al. Breast cancer stem cells. Breast Cancer 2010; 17:80–85. [DOI] [PubMed] [Google Scholar]
- 4.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 2008; 8:755–768. [DOI] [PubMed] [Google Scholar]
- 5.Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 2003; 100:3983–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramos P, Bentires-Alj M. Mechanism-based cancer therapy: resistance to therapy, therapy for resistance. Oncogene 2014; 34:3617–3626. [DOI] [PubMed] [Google Scholar]
- 7.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013; 19:1423–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 2013; 31:860–867. [DOI] [PubMed] [Google Scholar]
- 9.Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2010; 28:105–113. [DOI] [PubMed] [Google Scholar]
- 10.Pichelmayer O, Gruenberger B, Zielinski C, et al. Bevacizumab is active in malignant effusion. Ann Oncol 2006; 17:1853. [DOI] [PubMed] [Google Scholar]
- 11.Mullard A. New checkpoint inhibitors ride the immunotherapy tsunami. Nat Rev Drug Discov 2013; 12:489–492. [DOI] [PubMed] [Google Scholar]
- 12.Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep 2014; 15:1243–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cox TR, Erler JT. Molecular pathways: connecting fibrosis and solid tumor metastasis. Clin Cancer Res 2014; 20:3637–3643. [DOI] [PubMed] [Google Scholar]
- 14.Kakkad SM, Solaiyappan M, Argani P, et al. Collagen I fiber density increases in lymph node positive breast cancers: pilot study. J Biomed Opt 2012; 17:116017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakashima K, Shiina T, Sakurai M, et al. JSUM ultrasound elastography practice guidelines: breast. J Med Ultrasonics 2013; 40:359–391. [DOI] [PubMed] [Google Scholar]
- 16.Itoh A, Ueno E, Tohno E, et al. Breast disease: clinical application of US elastography for diagnosis. Radiology 2006; 239:341–350. [DOI] [PubMed] [Google Scholar]
- 17.Farmer P, Bonnefoi H, Anderle P, et al. A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat Med 2009; 15:68–74. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi M, Yamamoto Y, Ibusuki M, et al. Evaluation of tumor stiffness by elastography is predictive for pathologic complete response to neoadjuvant chemotherapy in patients with breast cancer. Ann Surg Oncol 2012; 19:3042–3049. [DOI] [PubMed] [Google Scholar]
- 19.Sadigh G, Carlos RC, Neal CH, et al. Accuracy of quantitative ultrasound elastography for differentiation of malignant and benign breast abnormalities: a meta-analysis. Breast Cancer Res Treat 2012; 134:923–931. [DOI] [PubMed] [Google Scholar]
- 20.Cho N, Moon WK, Kim HY, et al. Sonoelastographic strain index for differentiation of benign and malignant nonpalpable breast masses. J Ultrasound Med 2010; 29:1–7. [DOI] [PubMed] [Google Scholar]
- 21.Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 2013; 24:2206–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sueta A, Yamamoto Y, Yamamoto-Ibusuki M, et al. An integrative analysis of PIK3CA mutation, PTEN, and INPP4B expression in terms of trastuzumab efficacy in HER2-positive breast cancer. PLoS ONE 2014; 9:e116054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 2007; 25:118–145. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi M, Jono H, Shinriki S, et al. Clinical significance of CYLD downregulation in breast cancer. Breast Cancer Res Treat 2014; 143:447–457. [DOI] [PubMed] [Google Scholar]
- 25.Pickup MW, Laklai H, Acerbi I, et al. Stromally derived lysyl oxidase promotes metastasis of transforming growth factor-beta-deficient mouse mammary carcinomas. Cancer Res 2013; 73:5336–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans A, Rauchhaus P, Whelehan P, et al. Does shear wave ultrasound independently predict axillary lymph node metastasis in women with invasive breast cancer? Breast Cancer Res Treat 2014; 143:153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Youk JH, Gweon HM, Son EJ, et al. Shear-wave elastography of invasive breast cancer: correlation between quantitative mean elasticity value and immunohistochemical profile. Breast Cancer Res Treat 2013; 138:119–126. [DOI] [PubMed] [Google Scholar]
- 28.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer 2009; 9:108–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chamming's F, Latorre-Ossa H, Le Frere-Belda MA, et al. Shear wave elastography of tumour growth in a human breast cancer model with pathological correlation. Eur Radiol 2013; 23:2079–2086. [DOI] [PubMed] [Google Scholar]
- 30.Mouw JK, Yui Y, Damiano L, et al. Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nat Med 2014; 20:360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009; 139:891–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paszek MJ, Zahir N, Johnson KR, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005; 8:241–254. [DOI] [PubMed] [Google Scholar]
- 33.El-Haibi CP, Bell GW, Zhang J, et al. Critical role for lysyl oxidase in mesenchymal stem cell-driven breast cancer malignancy. Proc Natl Acad Sci USA 2012; 109:17460–17465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cox TR, Rumney RM, Schoof EM, et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature 2015; 522:106–110. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Mori R, Shaw TJ, Martin P. Molecular mechanisms linking wound inflammation and fibrosis: knockdown of osteopontin leads to rapid repair and reduced scarring. J Exp Med 2008; 205:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jono H, Lim JH, Chen LF, et al. NF-kappaB is essential for induction of CYLD, the negative regulator of NF-kappaB: evidence for a novel inducible autoregulatory feedback pathway. J Biol Chem 2004; 279:36171–36174. [DOI] [PubMed] [Google Scholar]
- 37.Chang JM, Moon WK, Cho N, et al. Breast mass evaluation: factors influencing the quality of US elastography. Radiology 2011; 259:59–64. [DOI] [PubMed] [Google Scholar]
- 38.Simpson ER, Brown KA. Obesity and breast cancer: role of inflammation and aromatase. J Mol Endocrinol 2013; 51:T51–T59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


