Abstract
The aim of this single-institutional 10-year retrospective study was to investigate the clinical pattern (incidence, type, timing, and location) of venous thromboembolism (VTE) in Chinese patients with gynecologic cancer.
Cases were identified by searching institutional Electronic Discharge Database. A comprehensive review of medical documentation was then performed to collect relevant data. The detection of VTE was symptom-triggered.
A total of 155 VTE events were identified out of 7562 cases over the past 10-year period in our hospital. The incidence of clinically significant VTE was 2.0% in gynecologic malignancy, with vulvar cancer (3.7%) and ovarian cancer (2.5%) being the high-risk types (P = 0.01, Chi-square test). Perioperative period (35.1%) and preoperation (29.1%) were the 2 incidence peaks. Seventeen cases of pulmonary embolism (PE) occurred prior to surgery. Ovarian cancer patients were more likely to present preoperative PE compared to other site of cancer (76.4%; P = 0.01, Chi-square test). More preoperative VTE cases were complicated by PE than those in the perioperative period (39.5% vs 17.3%, P = 0.02, Chi-square test). Bilateral lower extremity deep vein thrombosis (DVT) accounted for 32.6% and there existed a preponderance of left-sided DVT (47.5% vs 17.0%, ratio 2.79:1). Femoral vein (36.6%) was the most common location for DVT.
About 2.0% of the Chinese patients with gynecologic carcinoma developed clinical VTE, mostly during perioperative period and the time of diagnosis. The true incidence might have been under-estimated due to several reasons. The need for increased patient education and awareness of VTE is of importance.
INTRODUCTION
The relationship between cancer and venous thromboembolism (VTE) has long been investigated since Trousseau first described the possible association more than 100 years ago.1 VTE consists of deep vein thrombosis (DVT) and pulmonary embolism (PE) and is the second most common cause of deaths in patients with active cancer.2–4 It is well established that women with gynecologic malignancy are at high-risk for VTE due to a cancer diagnosis, advanced age, pelvic mass compressing the vasculature, lengthy surgery, and thrombogenic chemotherapy.5,6 The reported rate of DVT in patients with gynecologic malignancy ranged from 11% to 18% in literature, while the rate of PE between 1% and 2.6%.6–8
Several clinical risk factors have been suggested to be in association with cancer-related VTE, which can be broadly classified into patient-related (like ethnicity, age, and comorbidities), cancer-related (time after disease diagnosis, primary site, and histological type), and treatment-related factors (surgery and chemotherapy).9 Rates of VTE are lower in the Asian population than in other ethnicities.10,11 Besides, primary site distribution of gynecologic cancer patients in Asian country is different from that in Western counterpart. Therefore, we wondered if there was any difference of VTE pattern (incidence, type, timing, and location) between these 2 group patients. However, most published studies presented the characteristics of VTE in Western population, while data were relatively limited in the Chinese patients. Therefore, we felt it necessary to retrospectively analyze our own data from a tertiary referral center in Beijing, China.
The purpose of this study was quite straightforward as aforementioned. It was a single-institutional retrospective study to explore the clinical pattern of VTE cases in Chinese patients.
METHODS
Patients and Information
We conducted a 10-year retrospective study in Peking Union Medical College Hospital, which is a tertiary referral center while Obstetrics and Gynecology ranks the highest in China. Approval for the current study was obtained from the Institutional Review Board. We then performed a search of the Electronic Discharge Database. Each record comprises basic sociodemographic information, principle diagnosis, and clinicopathologic information. All the patients with gynecologic malignancy who also had VTE diagnosis (including DVT and PE) were identified during the time period of 12/31/2003 to 12/31/2013.
A comprehensive review of medical documentation was then carried out. The following data were abstracted from record including age at diagnosis, type of gynecologic malignancy, International Federation of Gynecology and Obstetrics (FIGO) stage, histology subtype, date and type of primary surgery, adjuvant treatment, VTE type and onset, thromboembolism location (side and specific vein), serum D-dimer level, body mass index (BMI, calculated as weight (kg)/[height (m)]2) and tumor status at the date of last contact.
In the present study, stage of diagnosis was designated based on the 1999 FIGO staging. Patients with gynecologic malignancies were thought to be the high-risk cases of VTE and asked to wear thromboembolic deterrent stockings postoperatively and treated with prophylactic anticoagulation therapy (low molecular weight heparin) from 48 hours after surgery to 14 days.
In our routine practice, 1 surgical specimen is usually reviewed by 2 pathologists (1 young and 1 senior doctor). A 3rd experienced pathologist will review the slides to confirm the diagnosis in some difficult cases or to resolve discrepancy. Diagnosis was mainly dependent on original pathology reports, and pathology review was not conducted in this study. Histologic subtype was classified according to the World Health Organization definitions.12
Detection of VTE Events
All VTE events were diagnosed using imaging triggered by patients’ symptoms. Diagnosis of VTE was based on clinical impression and imaging findings. Because VTE screening was not routinely performed, only clinically symptomatic thromboembolic events were identified. DVT was diagnosed by bilateral venous compression ultrasonography (for low extremity thrombus) and computerized tomography (for pelvic thrombus); PE was diagnosed by spiral computerized tomography pulmonary angiogram and ventilation perfusion lung scan. Patients with confirmed VTE were treated immediately by standard anticoagulation therapy.
Date of thromboembolic event was considered the date of diagnosis, which was further broadly classified by timing with respect to operation. Preoperative VTE included events either noted at diagnosis or between the interval of admission and surgery. Postoperative VTE comprised episodes observed in perioperative period (between surgery and commencement of primary therapy), during primary treatment, at the time of recurrence and follow-up visits. For patients without primary treatment, perioperative period was defined as the time interval from surgery to postoperation day 14.
Statistical Analysis
Statistical analysis was performed using SPSS statistical software (Version 17.0, SPSS, Inc, Chicago, IL). Microsoft Excel for Mac (Version 14.1.0, Microsoft, Seattle, WA) and GraphPad Prism (Version 5.0, GraphPad Software, Inc, La Jolla, CA) were used for figure illustration. Descriptive statistics were used to characterize the study. Categorical variables were compared by Chi-square test. A value of P < 0.05 was considered statistically significant and P value reported was 2-sided.
RESULTS
Over the 10-year period, there were 7562 cases of gynecologic malignancy in the electronic database upon discharge diagnosis. Of them, 155 (2.0%) patients experienced clinically apparent VTE events. Seven patients had concurrent ovarian and endometrial cancer and were not included in the following analysis.
Description of the Cohort
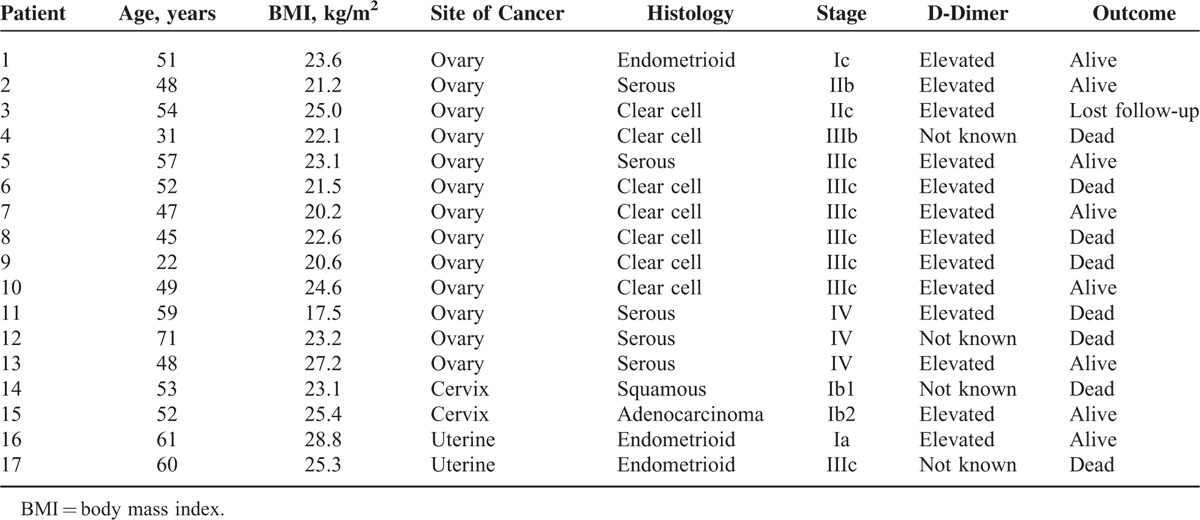
Table 1 outlines the basic information of the full cohort including age, BMI, serum D-dimer level, and FIGO stage. Thirty-seven percent (2828/7562) of the total cases were ovarian cancer, followed by cervical cancer (36.2%, 2738/7562), uterine cancer (21.6%, 1636/7562), vulvar cancer (2.9%, 219/7562), and vaginal cancer (1.9%, 141/7562). Seven concurrent ovarian/endometrial cancer cases were not involved in the table. Of the 148 patients included, mean age was 53.0 ± 11.6 years and 43 patients (29.1%) were older than 60 years. Mean BMI (BMI ± SD) was 24.0 ± 3.2 kg/m2 while 70 patients (47.3%) had a BMI higher than 24 kg/m2. A total of 83 patients (56.1%) had serum D-dimer measured at the time of VTE diagnosis. Normal serum D-dimer was observed in 27.7% (23/83) of the patients.
TABLE 1.
Demographic and Clinical Characteristics of Patients With Venous Thromboembolism
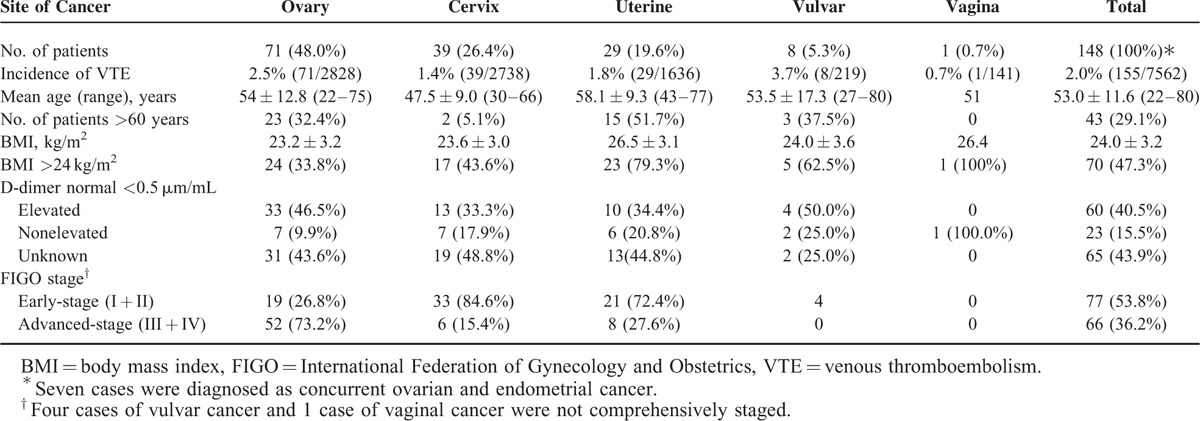
As to tumor stage, 73.2% of the ovarian carcinoma patients had late-stage disease (FIGO III + IV) at presentation while patients with cervical and uterine counterparts tended to have early disease in this cohort. We were interested in the issue whether or not there was any relationship between histology subclassification and VTE predisposition. Figure 1 illustrates number of VTE episodes in the entire cohort by histology subtype. As a whole, the major histology distribution of the tumors was as follows: squamous (25.0%, 37/148), serous (21.6%, 32/148), clear cell (20.9%, 31/148), endometrioid (16.2%, 24/148), and adenocarcinoma (6.1%, 9/148). Of note, clear cell carcinoma seemed to be VTE predisposing with 3 spikes seen in ovarian, cervical, and uterine cancer.
FIGURE 1.
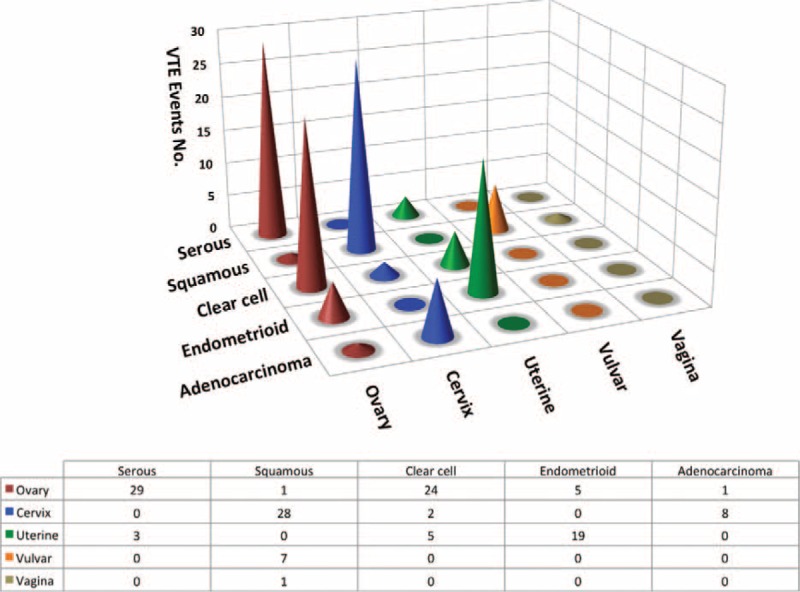
Number of venous thromboembolism (VTE) events in the gynecologic malignancy by histology subtype and site of cancer. The total number added to 133 instead of 148. The rest 15 cases were: 2 cases of ovarian cancer with mixed histology, 3 cases of mucinous ovarian cancer, 6 cases of ovarian cancer with other histology, 1 case of cervical cancer with other histology, 2 cases of uterine cancer with other histology, and 1 vulvar Paget's disease.
Clinical Patterns of VTE
As clearly seen from Table 1, vulvar cancer patients (3.7%) were most likely to develop VTE events, followed by ovarian cancer patients (2.5%), uterine cancer patients (1.8%), cervical cancer patients (1.4%), and vaginal cancer patients (0.7%) (P = 0.01, Chi-square test).
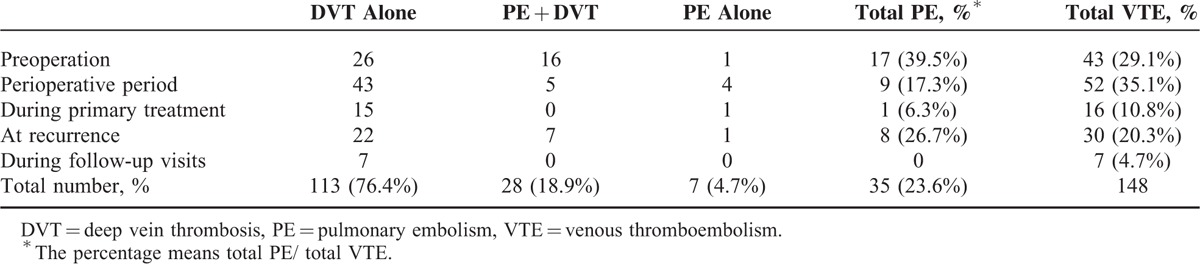
Table 2 further illustrates the timing and types of VTE events in the study. Thrombotic events tended to occur in perioperative period (35.1%) and preoperation (29.1%). Meanwhile, quite a few patients (20.3%) experienced VTE episode at recurrence. It was interesting to find that more VTE cases observed before surgery were complicated by PE than those in the perioperative interval (39.5% vs 17.3%, P = 0.02, Chi-square test).
TABLE 2.
Features of Venous Thromboembolism in Gynecologic Malignancy
Among 141 cases with DVT, one had thrombus in portal system including splenic, superior mesenteric vein, and portal vein, while 3 patients had lower extremity thrombus with no specific information available. A total of 137 DVT cases were included to further analyze specific location of thrombus. Interestingly, almost a half of the patients (67/141, 47.5%) had identifiable thrombus in the left side compared to 17.0% (24/141) in the right counterpart. Bilateral DVT was noted in a 3rd patient (46/141, 32.6%). Data on the location of the thrombotic event in DVT are presented in Figure 2. One patient might have thrombi in more than 1 vein. Thus as shown in the figure, the total number of involved veins are 224 in all the patients. The most common locations for DVT included femoral (82/224, 36.6%) and soleal vein (60/224, 26.8%). Pelvic vein (iliac and inferior vena cava) thrombus was found in 14.9% (21/141) of the patients.
FIGURE 2.
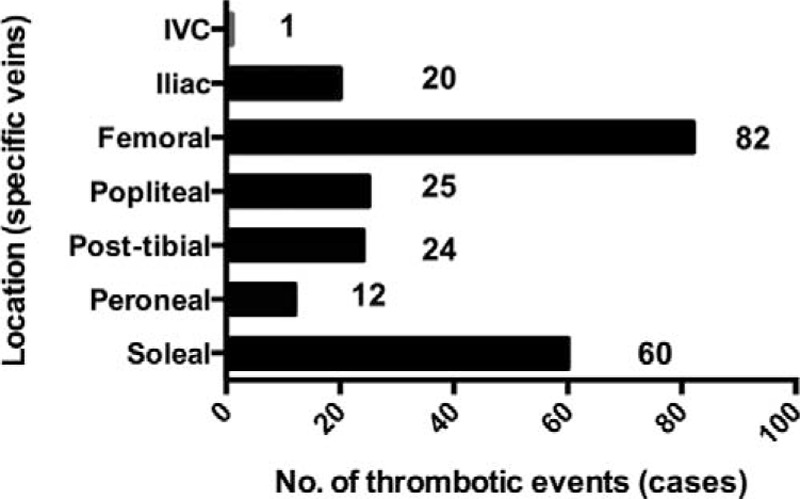
Anatomy of thrombus location of DVT. One patient might have thrombi in more than 1 vein. Thus, the total number of veins involved is 224. DVT = deep vein thrombosis, IVC = inferior vena cava.
We were particularly interested in preoperative PE cases in that more PE events were noted before surgery than other VTE timing as previously said. All the 17 cases were listed out in Table 3. The majority of the patients had ovarian carcinoma (13/17, 76.4%), followed by cervical cancer (2/17, 11.8%) and uterine cancer (2/17, 11.8%). We compared the percentage of ovary, cervix, uterine in both PE and non-PE/total populations and did a Chi-square test to show that P value was 0.01, which is statistically significant. As to ovarian cancer, most commonly recorded histology subtype was clear cell carcinoma (53.8%, 7/13). Serum D-dimer was elevated in all the patients except 4 with unknown level.
TABLE 3.
Clinical Features of the 17 Patients With Episode of Pulmonary Embolism Before Surgery
DISCUSSION
In the study, we evaluated the pattern of VTE cases in gynecologic malignancy, including incidence, timing, and type of VTE as well as anatomical location of DVT. The cumulative incidence of clinically apparent thromboembolism was about 2.0%, with vulvar cancer (3.7%) and ovarian cancer (2.5%) being the most commonly documented type. Thrombotic events were most likely to occur during perioperative period (35.1%) and at diagnosis (29.1%). VTE cases noted before surgery tended to be complicated by PE. The majority (76.4%) of the patients with preoperative PE were diagnosed as ovarian cancer. Concerning DVT, nearly a 3rd patients had bilateral thrombus and more patients developed left-sided thrombotic events with a left-to-right ratio of 2.79:1. Femoral and soleal vein involvement accounted for 36.6% and 26.8%, respectively.
The incidence in our series seemed to be lower than previously reported in published literature.5,7,8 Speaking of ovarian cancer alone, Rodriguez et al13 analyzed a large sample (n = 13,031) cases based on the California Cancer Registry, to find that the 24-month cumulative incidence was 5.2%. One plausible explanation for this disparity lies in ethnicity difference. Asian population has lower rates of VTE compared to Western people.10,11 However, according to the data in 2010, 14.9% of the population in California is Asian. Therefore, cautions should be taken when comparing our data with the California Cancer Registry data. In addition, we postulated that cases of VTE might have been missed in the institutional database and that we have under-estimated the true incidence especially in patients who live outside the city. Gynecologic Oncology Department in our institution is a well-known referral center and receives patients all over the country. Therefore, It is likely that not all VTE were captured as patients may have sought medical care at a facility close to their home.
Based on our data, vulvar cancer and ovarian cancer were the most common types complicated by VTE. In addition, ovarian cancer patients tended to present pulmonary emboli prior to surgery. In a recent investigation on solid tumors with PE from our hospital, Ma et al14 found that lung cancer patients constituted the largest proportion of the 120 cases (37.5%), followed by breast (9.2%) and ovarian (8.2%) cancer patients. It is reported that most VTE-related deaths are sudden fatal PE events.15,16 Fotopoulou et al17 pointed out that PE, but not DVT alone, was an independent negative prognostic factor. When we further assess all the cases of preoperative PE, we found that more than a half of the ovarian cancer patients had clear-cell histology. Our previous study,18 aimed to explore the prognostic implication of VTE in ovarian clear cell carcinoma, revealed that the total incidence was 14.5% and patients with VTE had grave survival. It might be a possibility that clear cell carcinoma predisposed patients to thrombogenic phenotype. Future studies and solid evidence are warranted to arrive at this conclusion.
When it comes to timing of VTE episodes, we noticed 2 incidence peaks in perioperative period (35.1%) and before operation (29.1%). Several authors have suggested a possible correlation between tumor aggressiveness and thrombotic events.19,20 A further peak in incidence albeit small was seen at disease recurrence (20.3%). VTE cases during primary treatment (mostly adjuvant chemotherapy) accounted for 10.8%. This point was of clinical significance in that more patients in our hospital and other institutions in China are now receiving postoperative chemotherapy in outpatient setting. Existing clinical guidelines recommend VTE prophylaxis for cancer patients through hospitalization,11,21–23 while recent updates to 2 guidelines suggesting that some outpatient chemotherapy patients could also benefit.22,23
Bilateral DVT occurred in 32.6% of the patients and left-sided thrombus was more commonly reported. This might be due to the anatomical reason that left common iliac vein was crossed over by the entire accompanying artery. In a study of 885 cases of lower extremity DVT, anatomic location was extensively investigated.24 It was concluded that the preponderance of left-sided DVT might be related to the high-frequency left common iliac vein involvement.24 A recent case–control radiology study also confirmed iliac vein compression as risk factor for left-sided DVT.25
Another important issue was that VTE events in the current study and also in our routine practice are mostly symptom-triggered. In other words, the prerequisite for detection is patient's sense of relevant symptom and sign. In spite of the well-known association between VTE and cancer, patients are woefully unaware of that risk and of warning signs and symptoms.11 One patient survey, targeting outpatients and active cancer population, revealed that less than half of the patients were aware of the increased risk of VTE associated with malignancy.26
There are several limitations of the current study. First, retrospective data collection inevitably leads to incomplete and ascertainment bias. Second, it is possible that the rates of VTE were underestimated partly in that only VTE cases recorded in Electronic Discharge Database were included in the study. Moreover, single-institutional study might not be representative of the whole country. Last, we only conducted a descriptive analysis of the clinical pattern of VTE occurrence, without any information regarding the prognostic implication.
CONCLUSION
The overall incidence of clinical VTE was 2.0% in gynecologic malignancy. Thrombotic events were likely to occur during perioperative period and the time of diagnosis. The majority of the patients with preoperative PE were diagnosed as ovarian cancer. Approximately a 3rd patient had bilateral thrombus and more patients developed left-sided DVT.
Footnotes
Abbreviations: BMI = body mass index, DVT = deep vein thrombosis, FIGO = International Federation of Gynecology and Obstetrics, PE = pulmonary embolism, VTE = venous thromboembolism.
SY and WZ contributed equally to this work.
Conception and design: SY, WZ, JY, DC, and KS. Collection and assembly of data: SY, WZ, HH, MW, JL, and KS. Manuscript writing: SY, WZ, JY, DC, and KS. Data analysis and interpretation, Final approval of manuscript: All authors.
The authors have no funding and conflicts of interest to declare.
REFERENCES
- 1.Khorana AA. Malignancy, thrombosis and Trousseau: the case for an eponym. J Thromb Haemost 2003; 1:2463–2465. [DOI] [PubMed] [Google Scholar]
- 2.Donati MB. Cancer and thrombosis. Haemostasis 1994; 24:128–131. [DOI] [PubMed] [Google Scholar]
- 3.Falanga A, Donati MB. Pathogenesis of thrombosis in patients with malignancy. Int J Hematol 2001; 73:137–144. [DOI] [PubMed] [Google Scholar]
- 4.Cohen AT, Nandini B, Wills JO, et al. VTE prophylaxis for the medical patient: where do we stand? – a focus on cancer patients. Thromb Res 2010; 125 Suppl 2:S21–S29. [DOI] [PubMed] [Google Scholar]
- 5.Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000; 160:809–815. [DOI] [PubMed] [Google Scholar]
- 6.Einstein MH, Pritts EA, Hartenbach EM. Venous thromboembolism prevention in gynecologic cancer surgery: a systematic review. Gynecol Oncol 2007; 105:813–819. [DOI] [PubMed] [Google Scholar]
- 7.Clark-Pearson DL, DeLong E, Synan IS, et al. A controlled trial of two low-dose heparin regimens for the prevention of postoperative deep vein thrombosis. Obstet Gynecol 1990; 75:684–689. [PubMed] [Google Scholar]
- 8.Clarke-Pearson DL, Coleman RE, Synan IS, et al. Venous thromboembolism prophylaxis in gynecologic oncology: a prospective, controlled trial of low-dose heparin. Am J Obstet Gynecol 1983; 145:606–613. [DOI] [PubMed] [Google Scholar]
- 9.Khorana AA, McCrae KR. Risk stratification strategies for cancer-associated thrombosis: an update. Thromb Res 2014; 133 Suppl 2:S35–S38. [DOI] [PubMed] [Google Scholar]
- 10.White RH, Keenan CR. Effects of race and ethnicity on the incidence of venous thromboembolism. Thromb Res 2009; 123 Suppl 4:S11–S17. [DOI] [PubMed] [Google Scholar]
- 11.Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2013; 31:2189–2204. [DOI] [PubMed] [Google Scholar]
- 12.Tavassoli FA, Devilee P. Pathology and genetics of tumours of the breast and female genital organs: Iarc; 2003. [Google Scholar]
- 13.Rodriguez AO, Wun T, Chew H, et al. Venous thromboembolism in ovarian cancer. Gynecol Oncol 2007; 105:784–790. [DOI] [PubMed] [Google Scholar]
- 14.Ma SQ, Lin Y, Ying HY, et al. Solid malignancies complicated with pulmonary embolism: clinical analysis of 120 patients. Chin Med J (Engl) 2010; 123:29–33. [DOI] [PubMed] [Google Scholar]
- 15.Gerotziafas GT, Samama MM. Prophylaxis of venous thromboembolism in medical patients. Curr Opin Pulm Med 2004; 10:356–365. [DOI] [PubMed] [Google Scholar]
- 16.Stroud W, Whitworth JM, Miklic M, et al. Validation of a venous thromboembolism risk assessment model in gynecologic oncology. Gynecol Oncol 2014; 134:160–163. [DOI] [PubMed] [Google Scholar]
- 17.Fotopoulou C, duBois A, Karavas AN, et al. Incidence of venous thromboembolism in patients with ovarian cancer undergoing platinum/paclitaxel-containing first-line chemotherapy: an exploratory analysis by the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group. J Clin Oncol 2008; 26:2683–2689. [DOI] [PubMed] [Google Scholar]
- 18.Ye S, Yang J, Cao D, et al. Characteristic and prognostic implication of venous thromboembolism in ovarian clear cell carcinoma: a 12-year retrospective study. PLoS One 2015; 10:e0121818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falanga A, Marchetti M, Vignoli A, et al. Clotting mechanisms and cancer: implications in thrombus formation and tumor progression. Clin Adv Hematol Oncol 2003; 1:673–678. [PubMed] [Google Scholar]
- 20.Falanga A. Thrombophilia in cancer. Semin Thromb Hemost 2005; 31:104–110. [DOI] [PubMed] [Google Scholar]
- 21.Mandala M, Falanga A, Piccioli A, et al. Venous thromboembolism and cancer: guidelines of the Italian Association of Medical Oncology (AIOM). Crit Rev Oncol Hematol 2006; 59:194–204. [DOI] [PubMed] [Google Scholar]
- 22.Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141 (2 Suppl):e195S–e226S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Streiff MB, Bockenstedt PL, Cataland SR, et al. Venous thromboembolic disease. J Natl Compr Canc Netw 2011; 9:714–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouriel K, Green RM, Greenberg RK, et al. The anatomy of deep venous thrombosis of the lower extremity. J Vasc Surg 2000; 31:895–900. [DOI] [PubMed] [Google Scholar]
- 25.Narayan A, Eng J, Carmi L, et al. Iliac vein compression as risk factor for left- versus right-sided deep venous thrombosis: case-control study. Radiology 2012; 265:949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sousou T, Khorana AA. Cancer patients and awareness of venous thromboembolism. Cancer Invest 2010; 28:44–45. [DOI] [PubMed] [Google Scholar]


