Supplemental Digital Content is available in the text
Abstract
End stage renal disease (ESRD) has been reported to be an important risk factor for systemic vascular disease. Retinal vein occlusion (RVO) is closely related with cardiovascular diseases; however, its association with ESRD had not been reported. The aim of the study was to investigate whether ESRD is a risk factor for RVO, including central retinal vein occlusion (CRVO) and branch retinal vein occlusion (BRVO). This population-based study is based on the longitudinal data from Taiwan National Health Insurance Research Database. The study cohort comprised 5344 patients with diagnosis of ESRD on hemodialysis or peritoneal dialysis during the period from January 1996 to December 2011. For each ESRD patient, we selected 20 non-ESRD patients matched on age and sex. Each ESRD patient and his/her controls were followed from the initiation of renal dialysis until either the diagnosis of RVO or censorship. Kaplan–Meier method was used to compare the hazard of RVO between cohorts. Stratified Cox proportional hazard models were applied to estimate the hazard ratio (HR) adjusted by the comorbidities of RVO including diabetes mellitus (DM), hypertension, hypercholesteremia, and hypertriglyceridemia. After stratifying by DM status, the statistics were applied again to examine the associations among the DM cohort and non-DM cohort.
The 16-year RVO cumulative incidence for ESRD cohort was 2-fold to the non-ESRD (1.01% vs 0.46%). After matching with age, sex, hypertension, and hypercholesteremia, the adjusted HR was 1.46 (95% confidence interval = 1.07–2.01, P value = 0.018). By further excluding patients with DM, the adjusted HR escalated to 2.43 (95% confidence interval = 1.54–3.83, P < 0.001). In contrast, there was no significant risk of ESRD on RVO in the DM patients (HR = 1.03). We conclude that among the non-DM patients, ESRD cases had significantly higher RVO rate than the non-ESRD, which indicates that ESRD maybe a potential risk factor for the development of RVO in nondiabetic patients.
INTRODUCTION
Retinal vein occlusion (RVO) is a major cause of visual loss, most in middle and elderly age groups.1 Clinical manifestations of RVO include visual deterioration, congested veins, retinal hemorrhage, retinal ischemia, extravasations of lipid, macular edema, and optic disc edema; neovascular glaucoma might develop in cases with wide nonperfused retina, which, if without treatment would lead to a painful, blind eye.2–5 Treatment of RVO included laser photocoagulation,6,7 administration of thrombolytic agents,8 and surgical interventions.9–11 Intravitreal injections of steroid or anti-VEGF agents were widely used in recent years,12–14 which are effective in reducing macular edema and improving visual acuity in most cases; however, most patients still suffer from repeated intravitreal injections.15 Risk factors for RVO include hypertension, hypercholesterolemia, myocardial infarction, diabetes mellitus (DM), and cerebral vascular accident.16–21 End stage renal disease (ESRD) has been reported to be an important risk factor for systemic vascular disease, such as cerebral vascular disease, coronary heart disease, carotid artery atherosclerosis.22,23 RVO closely related with cardiovascular diseases; however, its association with ESRD had not been reported. In this population based study, we aimed to investigate the relationship between ESRD and RVO to see whether ESRD with renal dialysis is an independent risk factor for RVO disease, including central retinal vein occlusion (CRVO) and branch retinal vein occlusion (BRVO).
MATERIALS AND METHODS
This study was conducted using a retrospective cohort analysis based on the Taiwan National Health Insurance (TNHI) Research Database. The TNHI program was initiated in 1995 and includes the medical records of >95% of Taiwan's hospitals contracted with TNHI. The coverage rate of the program increased from 92.4% in 1995 to over 96% in 2000, and exceeded over 98% of the Taiwanese population after the inclusion of the military forces in 2001. For research purposes, 1 million residents of Taiwan who were enrolled in the TNHI in 2005 were randomly selected by the National Health Research Institute to constitute the TNHI Research Database, which represents almost 4% of Taiwan's population and was re-sampled by the National Health Research Institute to be consistent with the structure of the general population with respect to sex and age, which make this longitudinal database quite representative of Taiwan's population.
The study cohort were patients newly diagnosed with ESRD on hemodialysis or peritoneal dialysis during the study period from January 1996 to December 2011. Each patient was ascertained by the criterion of receiving dialysis treatment for >2 times and treatment period of >3 months. Note that patients diagnosed with ESRD before the study start date, and diagnosed with RVO before ESRD (traced back to 1996 when the NHI database became operational) were excluded in advance to limit the study to newly diagnosed cases. After these exclusions, a total of 5344 patients with ESRD were included in the study cohort. For each patient on dialysis, roughly 20 patients who matched the age and sex were randomly selected from the patients out of the rest of the 1 million sample who had never been diagnosed with ESRD during the studied period. As a result, a total of 99,440 patients were assigned to a comparison cohort (some older ESRD cases might have had fewer controls to be matched with). In each matched stratum, an ESRD patient and his/her matched non-ESRD controls were followed up from the first day of receiving dialysis for the ESRD case until either the diagnosis of RVO or censorship (ie, termination of insurance or study period). In such way, the starting follow-up time for an ascertained ESRD and his/her non-ESRD controls is aligned. All cases in the database were counted only once. Before analysis, temporal consistency among the dates of birth, diagnosis, and clinical visits of each patient was checked to ensure accuracy. The matching and subsampling were conducted by using SAS 9.3 (SAS Institute Inc., NC).
We took into account the effects of the comorbidities of RVO, which includes DM, hypertension, hypercholesteremia, and hypertriglyceridemia. DM and hypertension were ascertained by >3 clinical diagnoses or at least 1 hospitalization. Hypercholesteremia and hypertriglyceridemia were ascertained by >2 clinical diagnoses or at least 1 hospitalization. A patient is then considered having a comorbidity if the first diagnosed date of that comorbidity is before the follow-up (ie, first ESRD diagnosis.) The International Classification of Diseases code version 9 (ICD-9) codes for the diseases are listed in the Appendix, http://links.lww.com/MD/A517.
Due to the dependence between the case and his/her matched controls within each stratum, the Mantel–Haenszel odds ratio (ORMH) instead of regular odds ratio was used to separately measure the univariate association between ESRD and RVO, as well as ESRD and CRVO and BRVO. Kaplan–Meier plots and log-rank tests were used to test the differences in the time of developing RVO between the ESRD and non-ESRD cohorts. For the multivariate analysis, stratified Cox proportional hazard models were applied to examine the association among ESRD on dialysis and RVO, adjusting for potential confounding factors that include DM, hypertension, hypercholesteremia, and hypertriglyceridemia. If a comorbidity was found to have versatile effects on RVOs, we further repeated the above analysis stratified by the status of that comorbidity, to explore its interaction effect with ESRD. The inclusion/exclusion of patients was shown in supplementary Figure, http://links.lww.com/MD/A517.
The study used only the encrypted chart records, and had been approved by the National Changhua University institution review board with IRB no. 102001 for not using informed consent.
RESULTS
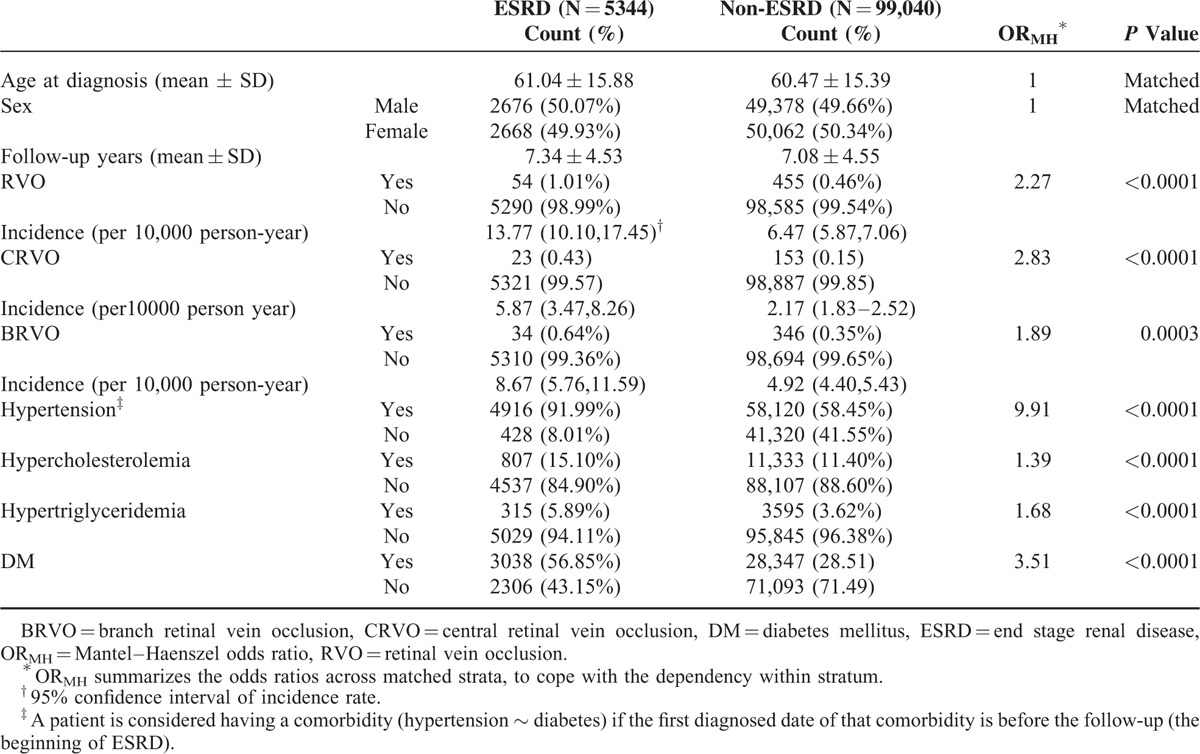
Table 1 shows that among the 5344 ESRD patients, 54 had developed RVO, including 23 CRVO and 34 BRVO (with 3 patients who had both). Among the 99,440 matched control patients, 455 had developed RVO (153 CRVOs and 346 BRVOs).
TABLE 1.
Distribution of RVOs and Comorbidities Between ESRD and Non-ESRD
ESRD is Associated With Higher Risk of CRVO
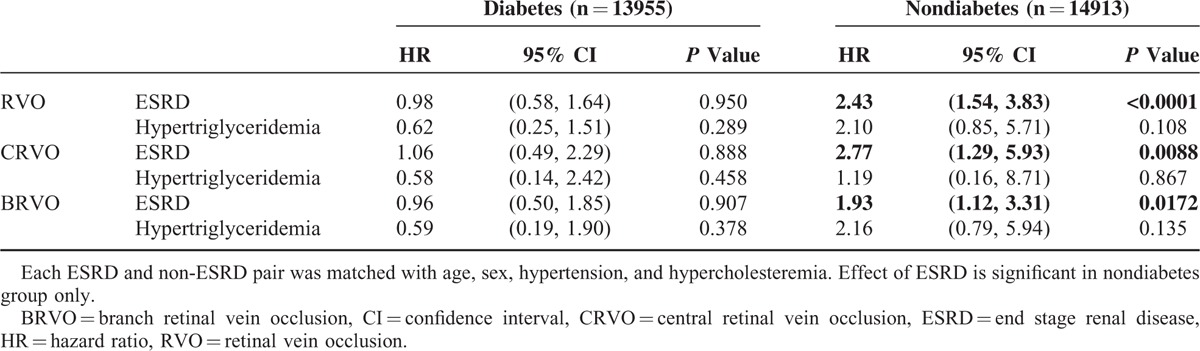
Table 1 also lists the univariate odds ratio ORMH of RVO summarized from the 16-year study period. The ORMH of RVO for the ESRD patients on dialysis was 2.27-fold that for the non-ESRD patients, with P value < 0.0001 from the log-rank test. The Kaplan–Meier plot in Figure 1A shows the gap of time to RVO-event between ESRD and non-ESRD. After adjusting for all comorbidities in a stratified Cox regression (Table 2), the hazard ratio (HR) remained significant at 1.50 (95% confidence interval [CI]:1.13–1.99) with a P value = 0.006. As hypertension and hypercholesteremia are significant, we further randomly took the 1-to-10 subsamples of ESRD and non-ESRD that matched the status of these comorbidities and rerun the stratified Cox regression. The results remain significant, with HR as 1.46 (95% CI:1.07–2.01) with a P value = 0.018, also significant in the model was diabetes.
FIGURE 1.
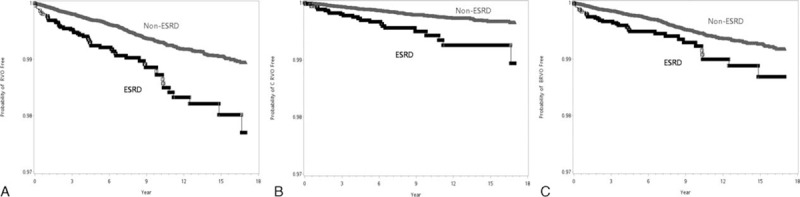
Kaplan–Meier plots. Time to the disease events in ESRD groups and non-ESRD group. A, RVO-event (either CRVO or BRVO); B, CRVO-event; C, BRVO-event. BRVO = branch retinal vein occlusion, CRVO = central retinal vein occlusion, ESRD = end stage renal disease, RVO = retinal vein occlusion.
TABLE 2.
Results of Cox Regression for RVO CRVO and BRVO
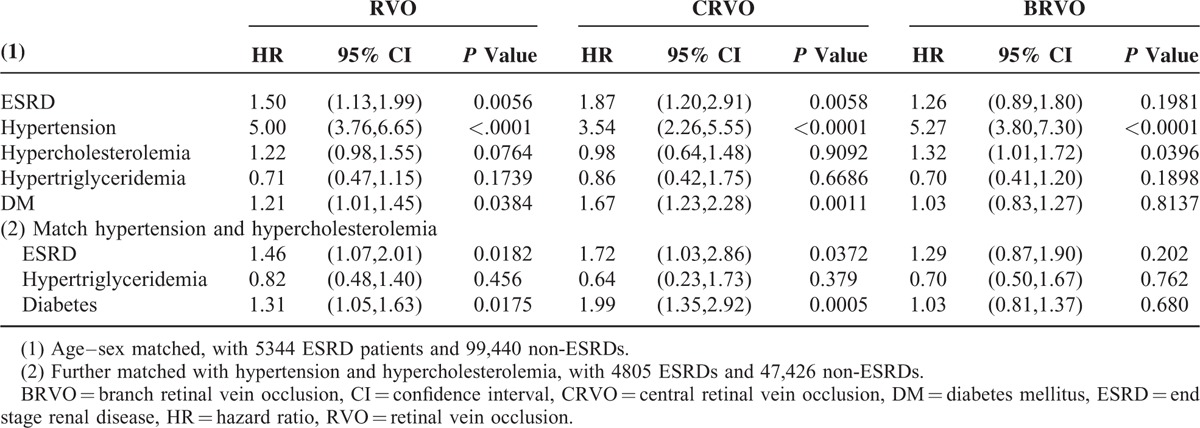
From separate analysis of CRVO and BRVO, the unadjusted ORMH were 2.81 and 1.83, with P values < 0.0001 and 0.0006, respectively (Table 1 and Figure 1B,C), whereas the adjusted HRs by Cox regression were reduced to 1.87 (CI: 1.20–2.91) and 1.26 (CI: 0.89–1.80), with P values of 0.0006 and 0.2, respectively (Table 2, top). After further matching with hypertension, and hypercholesteremia, the adjusted HRs were 1.72 (CI: 1.03–2.86) and 1.29 (CI: 0.87–1.90), with P values 0.037 and 0.2, respectively (Table 2, bottom). The effect of dialysis on BRVO was not as significant as that on CRVO in the multivariate analysis.
To further elaborate the interaction effect between diabetes and dialysis, we conducted a separate analysis for the diabetes group (3038 ESRD cases + matched control), and nondiabetes group (2306 ESRD cases + matched control).
Analysis Stratified by Diabetes
Table 3 shows that after matching with age, sex, hypertension, and hypercholesteremia, the ESRD patients with diabetes are roughly 2.7 years older than the ESRDs without diabetes. For the diabetes group, the univariate ORMH of RVO for ESRD to non-ESRD, as well as that of CRVO and BRVO were all not significantly different from 1. It is interesting to observe that ESRD group had the lower prevalence of comorbidity than non-ESRD group among diabetic patients, but had higher comorbidity prevalence among nondiabetic patients. DM seems to have some interaction effect with the other 3 comorbidities on ESRD. Therefore in Table 4, we did separate analyses for DM and non-DM with significant comorbidities being matched to eliminate the unbalancing.
TABLE 3.
Distribution of RVOs and Comorbidities Between ESRD and non-ESRD Stratified by Diabetes and Nondiabetes
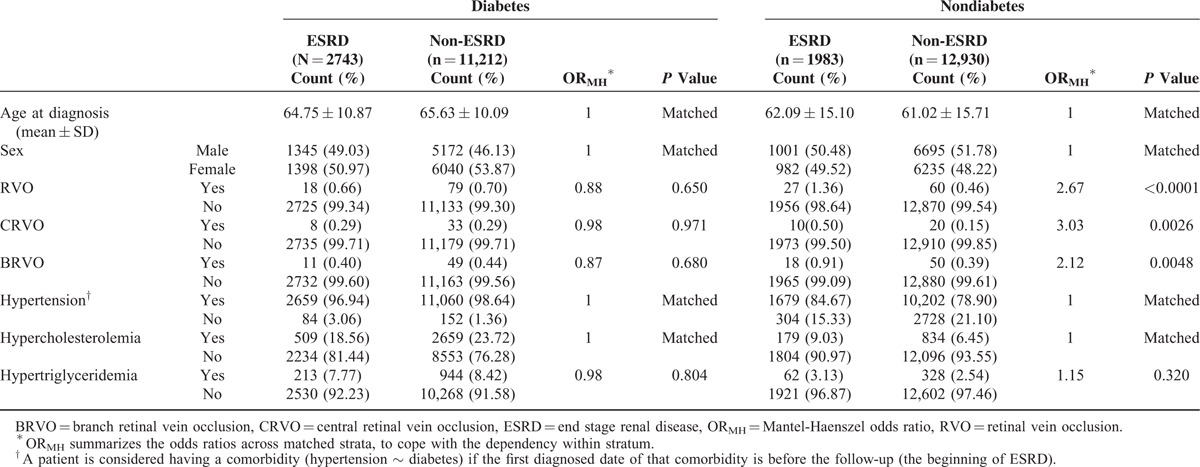
TABLE 4.
Results of Cox Regression for RVO, CRVO, and BRVO Stratified by Diabetes Status
The result remained nonsignificant by Cox regression in Table 4, with HRs 0.98, 1.06, and 0.96 for RVO, CRVO, and BRVO, respectively. In contrast, for the nondiabetes group, the univariate ORMH of RVO was 2.67 for ESRD to non-ESRD, and that of CRVO and BRVO were 3.03 and 2.12, wherein all were significant with P values < 0.0001, 0.0026, and 0.0048, respectively (Table 3, right).
The Kaplan–Meier plots in Figure 2A–C show the difference of time to RVO-, CRVO-, and BRVO-event between ESRD and non-ESRD patients without diabetes. The results did not change even after adjusting for the comorbidity effects of hypertriglyceridemia using a Cox regression. The adjusted HR of RVO as a whole was 2.43 (CI: 1.54, 3.83), with a P value < 0.0001, wherein the HR from the stratified analysis was 2.77 (CI: 1.29, 5.93) for CRVO and 1.93 (CI: 1.12, 3.31) for BRVO, with P values 0.0088 and 0.017, respectively (Table 4).
FIGURE 2.
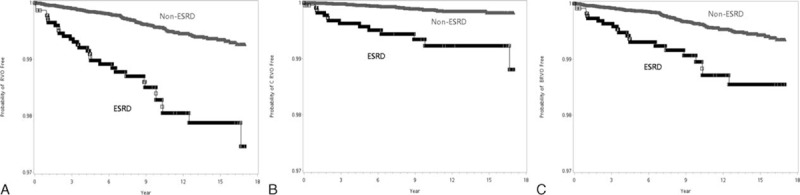
Separate analysis for nondiabetes patients. Kaplan–Meier plots for time to the disease events in ESRD groups and in non-ESRD group. A, RVO-event (either CRVO or BRVO); B, CRVO-event; C, BRVO-event BRVO = branch retinal vein occlusion, CRVO = central retinal vein occlusion, ESRD = end stage renal disease, RVO = retinal vein occlusion.
Assessment and Bias-Correction for Rare Event
We had repeated the random sampling process while matching the age, sex, hypertension, and hypercholesteremia between ESRD and non-ESRD, and the results came out very similar. There is a potential bias in a maximal likelihood estimate when it is applied to data of small sample sizes and with rare events. In our analysis, the Cox model used the so-called partial-likelihood. Although our sample size was large, the event (RVO) was relatively rare. For this reason, we compared our results from the Cox regression using default settings with that from Cox regression using exact estimation and Cox regression using Firth penalized maximal likelihood, which are known for their capacity to correct the bias.24 We found that the estimated HRs from these computational extensive methods were almost the same as the those from the stratified Cox regression in Table 2. We were then convinced that the bias due to the rareness of the RVO was negligible in this study.
DISCUSSION
The correlation between retinal venous occlusive disease and some systemic disease has been well established16–21; however, the relationship between ESRD and RVO has only been reported in a few sporadic studies. In 1 study from Japan,5 chronic kidney disease was found to be an independent risk factor for RVO, after matching and adjusting for other confounding factors including DM, blood pressure, serum cholesterol, etc. In the report of the Beaver Dam Eye Study,21 a 60% higher risk of RVO was observed in persons with elevated serum creatinine levels (1.4 mg/dL); however, in those studies, BRVO and CRVO were taken together as a single-disease group. In our population-based study, ESRD was noted as a potential risk factor for RVO, for both CRVO and BRVO (Table 1); however, after adjusting for other comorbidities, a significant difference was only noted in CRVO, but not BRVO in the Cox-regression model (Table 2). After stratifying DM status, the analysis showed a further increased significance of ESRD as a risk factor in RVO, CRVO, and BRVO in the ESRD cohort, and in the Cox regression model, the statistics became significant, for not only CRVO, but also for BRVO (Table 4) after adjusting for other comorbidities.
The association of ESRD and RVO might be multifactorial. Arteriosclerosis is widely present in patients with ESRD.22 The possible mechanisms for arteriosclerosis include chronic inflammation, alteration of extracellular matrix deposition, advanced glaycation end products, elevated aldosterone level, and disordered bone marrow minerization.22,23 As stiff arterial wall is one of the major pathogenic factors for both BRVO and CRVO,25,26 it is of no surprise that patients with ESRD had an increased risk of RVO. In addition to arteriosclerosis, hypercoagulable status has long been noted in patients with ESRD.27,28 The elevated level prothrombin fragment, thrombin-antithrombin complex, and homocysteine in plasma observed in patients with ESRD29–33 were also risk factors for RVO.25–37 Moreover, dialysis including both hemodialysis or peritoneal dialysis might stimulate the coagulation activity even more,33 which might further increase the risk of RVO in ESRD patients.
We noted in this study that in ESRD patients, the HR of CRVO is higher than that of BRVO. Although both are venous occlusive diseases, CRVO and BRVO are somewhat different in the pathogenesis. Whereas BRVO mainly happens in patients >40 years of age with stiff arterial wall,19,25 CRVO might also happen in young adults, who have not been noted to have arteriosclerosis.26 In addition to the common risk factors including arteriosclerosis, hypertension, hyperlipidemia, and DM for both CRVO and BRVO,16–21 other risk factors that might possibly also be correlated with BRVO, such as hypercoagulable status, blood hyperviscosity, and open angle glaucoma, seem to be more associated with CRVO.19 As elevated intraocular pressure during dialysis might happen in some predisposed patients38 and hypercoagulable blood status is common in ESRD patients, ESRD appears to be more of a risk factor for CRVO than BRVO.
It is interesting to note that the level of significance for developing RVO became more prominent in ESRD patients after excluding DM patients. This might be explained by several reasons. First, DM itself is a risk factor for CRVO and BRVO. It is possible that the damaging effect of DM on vasculature is so predominant that nullifies the additive deleterious effect of ESRD. Second, patients with vascular nephropathy on renal dialysis have been shown to have significantly higher serum concentration of prothrombin fragment as compared with their counterparts with diabetic nephropathy,33 thus indicating that dialysis might more strongly affect the coagulation profile in the non-DM ESRD patients.
The strength of this study is the large number of ESRD patients as a case cohort, and the long follow-up duration. On the contrary, the limited number of cases developing RVO makes the statistics weaker; further, this analysis was based on insurance claim data without funds photographic recordings, and for this reason, some patients with asymptomatic BRVO might not have been recorded and the incidence of BRVO might have been underestimated; however, the chance of underestimating the asymptomatic BRVO is likely to be similar in both the case and the control cohort, which might have only minimally affected the final results.
We used both matching and direct adjustment on 4 comorbidities (DM, hypertension, hypercholesteremia, and hypertriglyceridemia) in addition to age and sex for balancing the health conditions between the ESRD and non-ESRD groups; however, some residual confounding might still exist and potential unmeasured confounders such as atherosclerosis were not taken into account, which are the nature limitation of this retrospective study.
In conclusion, ESRD is a risk factor for CRVO. After stratifying DM status, it became a risk factor for both CRVO and BRVO; however, for DM patients, ESRD did not have the additive risk of developing RVO, either for CRVO or BRVO.
Footnotes
Abbreviations: BRVO = branch retinal vein occlusion, CI = Confidence Interval, CRVO = central retinal vein occlusion, DM = diabetes mellitus, ESRD = end stage renal disease, HR = hazard ratio, RVO = retinal vein occlusion, TNHI = Taiwan National Health Insurance.
The research is partially supported by Ministry of Science and Technology (MoST 103-2633-B-018-001).
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrence and demographic characteristics. Am J Ophthalmol 1994; 117:429–441. [DOI] [PubMed] [Google Scholar]
- 2.Wolter JR. Retinal pathology after central retinal vein occlusion. Br J Ophthalmol 1961; 45:683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Central Vein Occlusion Study Group. Natural history and clinical management of central retinal vein occlusion. Arch Ophthalmol 1997; 115:486–491. [DOI] [PubMed] [Google Scholar]
- 4.Hayreh SS. Prevalent misconceptions about acute retinal vascular occlusive disorders. Prog Retin Eye Res 2005; 24:493–519. [DOI] [PubMed] [Google Scholar]
- 5.McIntosh RL, Rogers SL, Lim L, et al. Natural history of central retinal vein occlusion: an evidence-based systematic review. Ophthalmology 2010; 117:1113–1123. [DOI] [PubMed] [Google Scholar]
- 6.Laatikainen L, Kohner EM, Khoury D, et al. Panretinal photocoagulation in central retinal vein occlusion: a randomised controlled clinical study. Br J Ophthalmol 1977; 61:741–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAllister IL, Constable IJ. Laser-induced chorioretinal venous anastomosis for treatment of non-ischemic CRVO. Arch Ophthalmol 1995; 113:456–462. [DOI] [PubMed] [Google Scholar]
- 8.Weiss JN. Treatment of central retinal vein occlusion by injection of tissue plasminogen activator into a retinal vein. Am J Ophthalmol 1998; 126:142–144. [DOI] [PubMed] [Google Scholar]
- 9.Opremcak EM, Bruce RA, Lomeo MD, et al. Radial optic neurotomy for central retinal vein occlusion: a retrospective pilot study of 11 consecutive cases. Retina 2001; 21:408–415. [DOI] [PubMed] [Google Scholar]
- 10.Chen SN, Huang YC. Full-thickness retinochoroidal incision in the management of central retinal vein occlusion. J Ophthalmol 2015; 2015:853539.doi: 10.1155/2015/853539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mason J, 3rd, Feist R, White M, Jr, et al. Sheathotomy to decompress branch retinal vein occlusion: a matched control study. Ophthalmology 2004; 111:540–545. [DOI] [PubMed] [Google Scholar]
- 12.Gewaily D, Greenberg PB. Intravitreal steroids versus observation for macular edema secondary to central retinal vein occlusion. Cochrane Database Syst Rev 2009; CD007324.doi(1):CD007324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nghiem-Buffet S, Cohen SY. Retinal vein occlusion: anti-VEGF treatments. J Fr Ophtalmol 2009; 32:679–686. [DOI] [PubMed] [Google Scholar]
- 14.Braithwaite T, Nanji AA, Greenberg PB. Anti-vascular endothelial growth factor for macular edema secondary to central retinal vein occlusion. Cochrane Database Syst Rev 2010; CD007325.doi(10):CD007325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brynskov T, Kemp H, Sørensen TL. Intravitreal ranibizumab for retinal vein occlusion through 1 year in clinical practice. Retina 2014; 34:1637–1643. [DOI] [PubMed] [Google Scholar]
- 16.Zhou JQ, Xu L, Wang S, et al. The 10-year incidence and risk factors of retinal vein occlusion: the Beijing eye study. Ophthalmology 2013; 120:803–808. [DOI] [PubMed] [Google Scholar]
- 17.Newman-Casey PA, Stem M, Talwar N, et al. Risk factors associated with developing branch retinal vein occlusion among enrollees in a United States managed care plan. Ophthalmology 2014; 121:1939–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim LL, Cheung N, Wang JJ, et al. Prevalence and risk factors of retinal vein occlusion in an Asian population. Br J Ophthalmol 2008; 92:1316–1319. [DOI] [PubMed] [Google Scholar]
- 19.Kolar P. Risk factors for central and branch retinal vein occlusion: a meta-analysis of published clinical data. J Ophthalmol 2014; 2014:724780.doi: 10.1155/2014/724780. Epub 2014 Jun 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arakawa S, Yasuda M, Nagata M, et al. Nine-year incidence and risk factors for retinal vein occlusion in a general Japanese population: the Hisayama Study. Invest Ophthalmol Vis Sci 2011; 52:5905–5909. [DOI] [PubMed] [Google Scholar]
- 21.Klein R, Moss SE, Meuer SM, et al. The 15-year cumulative incidence of retinal veinocclusion: the Beaver Dam Eye Study. Arch Ophthalmol 2008; 126:513–518. [DOI] [PubMed] [Google Scholar]
- 22.Moody WE, Edwards NC, Chue CD, et al. Arterialdisease in chronic kidney disease. Heart 2013; 99:365–372. [DOI] [PubMed] [Google Scholar]
- 23.Chue CD, Townend JN, Steeds RP, et al. Arterial stiffness in chronic kidney disease: causes and consequences. Heart 2010; 96:817–823. [DOI] [PubMed] [Google Scholar]
- 24.Heinze G, Schemper M. A solution to the problem of monotone likelihood in Cox regression. Biometrics 2001; 57:114–119. [DOI] [PubMed] [Google Scholar]
- 25.Rehak J, Rehak M. Branch retinal vein occlusion: pathogenesis, visual prognosis, and treatment modalities. Curr Eye Res 2008; 33:111–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parodi MB, Di Crecchio L. Hyperhomocysteinemia in central retinal vein occlusion in young adults. Semin Ophthalmol 2003; 18:154–159. [DOI] [PubMed] [Google Scholar]
- 27.Anavekar NS, McMurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med 2004; 351:1285–1295. [DOI] [PubMed] [Google Scholar]
- 28.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 29.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney diseases a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003; 108:2154–2169. [DOI] [PubMed] [Google Scholar]
- 30.Leskinen Y, Groundstroem K, Virtanen V, et al. Risk factors for aorticath erosclerosis determined by transesophageal echocardiography in patients with CRF. Am J Kidney Dis 2003; 42:277–285. [DOI] [PubMed] [Google Scholar]
- 31.Llach F. Hypercoagulability, renal vein thrombosis, other thrombotic complications of nephrotic syndrome. Kidney Int 1985; 36:272–2792. [DOI] [PubMed] [Google Scholar]
- 32.Vaziri ND, Branson HE, Ness R. Changes of coagulation factors in nephrotic syndrome. Am J Med Sci 1980; 280:167–171. [DOI] [PubMed] [Google Scholar]
- 33.Ambühl PM, Wüthrich RP, Korte W, et al. Plasmahypercoagulability in hemodialysis patients: impact of dialysis and anticoagulation. Nephrol Dial Transplant 1997; 12:2355–2364. [DOI] [PubMed] [Google Scholar]
- 34.Suliman ME, Stenvinkel P, Bárány P, et al. Hyperhomocysteinemia and its relationship to cardiovascular disease in ESRD: influence of hypoalbuminemia, malnutrition, inflammation, and diabetes mellitus. Am J Kidney Dis 2003; 41:S89–S95. [DOI] [PubMed] [Google Scholar]
- 35.Brown BA, Marx JL, Ward TP, et al. Homocysteine: a risk factor for retinal venous occlusive disease. Ophthalmology 2002; 109:287–290. [DOI] [PubMed] [Google Scholar]
- 36.Brian C, Annette K, Tien W, et al. Homocysteine and retinal vein occlusion: a population-based study. Am J Ophthalmol 2005; 139:181–182. [DOI] [PubMed] [Google Scholar]
- 37.Incorvaia C, Parmeggiani F, Costagliola C, et al. The heterozygous 20210 G/A genotype prevalence in patients affected by central and branch retinal vein occlusion: a pilot study. Graefes Arch Clin Exp Ophthalmol 2001; 239:251–256. [DOI] [PubMed] [Google Scholar]
- 38.Minguela I, Andonegui J, Aurrekoetxea B, et al. Prevention of intraocular pressure elevations during hemodialysis. Am J Kidney Dis 2000; 36:197–198. [DOI] [PubMed] [Google Scholar]


