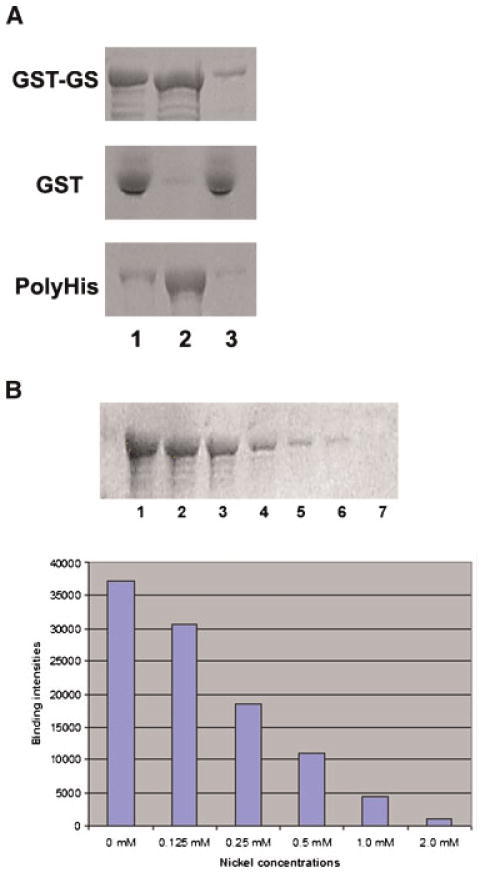
Figure 2.
Glutamate–ammonia ligase is a Ni2+ binding protein. A: Recombinant 68 kDa GST–GLUL protein was prepared in bacteria and analyzed for its capability to bind nickel by incubation with Ni-NTA agarose beads. GST–GS, recombinant 68 kDa GST–GLUL protein. GST, GST protein as a negative control. PolyHis, a poly(His)-tagged sperm tail protein used as a positive control. Lane 1, input protein; lane 2, proteins bound to Ni-NTA agarose beads; lane 3, unbound material (flow-through). B: Ni2+ competes with glutamate–ammonia ligase binding to Ni-NTA agarose beads. GST–GLUL recombinant protein was incubated with different amounts of nickel sulfate before incubation with Ni-NTA agarose beads. Then, GST–GLUL and nickel were incubated with Ni-NTA agarose beads and the bound protein was analyzed by SDS–PAGE. Top panel: Lane 1, 0 mM Ni2+; lanes 2–6, 0.125, 0.25, 0.50, 1.0, and 2.0 mM Ni2+, respectively. Bottom panel: Quantitation of the results is shown as a graph. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com]