Abstract
Apoptosis inhibitor of macrophage (AIM) is initially reported to protect macrophages from apoptosis. In this study, we determined the effect of AIM on the macrophage-derived tumor, histiocytic sarcoma cell lines (HS) of dogs. Five HS and five other tumor cell lines were used. When recombinant canine AIM was applied to non-serum culture media, cell numbers of all the HS and two of other tumor cell lines decreased dose-dependently. The DNA fragmentation, TUNEL staining and flow cytometry tests revealed that AIM induced both of apoptosis and cell cycle arrest in the HS. Although AIM is known as an apoptosis inhibitor, these results suggest that a high dose of AIM could have an opposite function in HS and some tumor cell lines.
Keywords: CD5L, histiocytic sarcoma, immuno competent cell, scavenger receptor cysteine-rich superfamily
Histiocytic sarcoma is a malignant tumor derived from histiocytic cell lineages, such as macrophages and dendritic cells [3]. Because of its rapid cell proliferation, multidrug resistance and high metastatic potential, histiocytic sarcoma has a generally poor response to therapy [8]. Although lomoustine (CCNU) or doxorubicin is mainly used for the treatment [13, 17], dogs with histiocytic sarcoma usually exhibit poor treatment outcomes and median survival time from diagnosis is reported to be 43 days (range 2–360 days) [14]. Therefore, new drugs or target proteins are currently being investigated for the effective treatment of this disease.
Apoptosis inhibitor of macrophage (AIM), also called CD5L, was identified in macrophages of mice [10]. It is a member of the scavenger receptor cysteine-rich (SRCR) superfamily which is characterized by the repeats of a highly homologous cysteine-rich domain [2]. As its name suggests, AIM is initially found as an inhibitor of apoptosis of macrophage itself [10]. Moreover, it has been found that AIM facilitates survival of foamed macrophages in atherosclerosis [12]. While several other functions of AIM on other cells or tissues have been also elucidated, the effect of AIM in the histiocytic sarcoma remains unknown. Hence, in this study, we evaluated cell viability, apoptosis and cell cycle of histiocytic sarcoma cells affected by AIM.
Five canine histiocytic sarcoma cell lines (CHS-4, CHS-5, CHS-7, MHT-2 [1] and DH82 [18]), two canine mammary gland tumor cell lines (CHMp and CTBp [16]), two canine melanoma cell lines (CMM1 [11] and CMM 11) and a canine transitional cell carcinoma cell line (TCCUB) were used. DH82 originated from macrophage, while the other histiocytic sarcoma cell lines were unclassified, although they have phagocytic function [1]. CHS-4, CHS-5, CHS-7 and MHT-2 were given by Professor Makoto Bonkobara, the Nippon Veterinary and Life Science University (Tokyo, Japan). CTBp, CHMp, CMM1, CMM 11 and TCCUB were gifted from Professor Ryohei Nishimura, the University of Tokyo (Tokyo, Japan). All the cell lines were appropriately cultured according to the previous reports [1, 11, 16, 18].
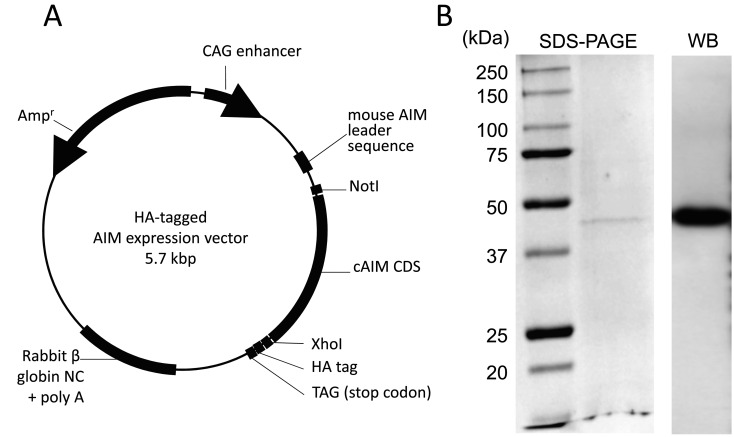
Recombinant canine AIM (rcAIM) was synthesized by transfection of the expression vector into CHS-5 cells. The expression vector was constructed based on the HA-tagged pCAGGS plasmid (Fig. 1A). The plasmid was kindly gifted from Professor Toru Miyazaki, the University of Tokyo. AIM cDNA was derived from a spleen of a healthy Beagle. Gene transfection was performed by the lipofection method using TransIT-LT1 transfection reagent (TaKaRa, Otsu, Japan). After gene transfection, HA-tagged AIM was collected from the cell lysate using Anti-HA Affinity Matrix (Roche, Basel, Switzerland). The flow-through elution solvent was replaced with PBS by 24 hr of dialysis at 4°C. The solution was condensed by ultrafiltration using Centriprep 30 kDa (Millipore, Bedford, MA, U.S.A.). Obtained protein was identified with rcAIM by its size and HA antigenicity using SDS-PAGE with Coomassie brilliant blue (CBB) staining and Western blotting (Fig. 1B).
Fig. 1.
Production of HA-tagged rcAIM. (A) HA-tagged rcAIM expression vector was cloned into the modified plasmid vector pCAGGS. It contains cytomegalovirus enhancer, chicken β-actin promoter, rabbit β-globin exons and intron, HA polypeptide, and mouse AIM leader sequence. (B) Recombinant protein was observed on the polyacrylamide gel stained CBB (left) and the membrane of Western blotting for HA (right).
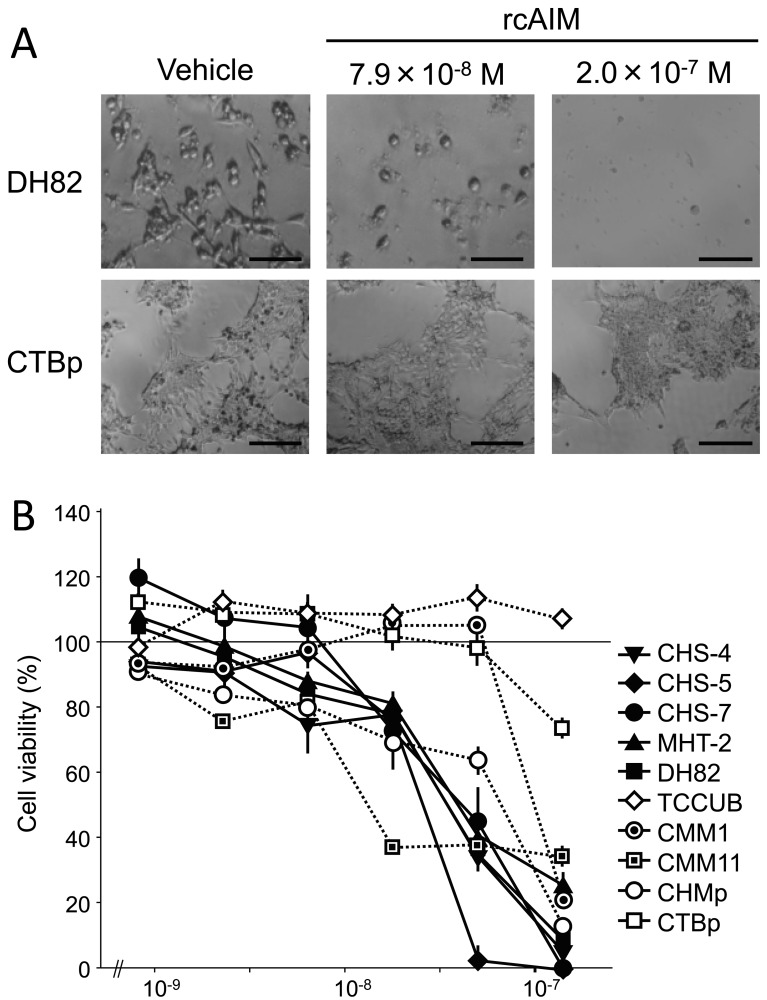
To observe the effect of AIM on cell viability in canine tumor cell lines, five histiocytic sarcoma and five other tumor cell lines as mentioned above were used. Each cell line was seeded in 96-well plates at a density of 1.0 × 104 cells with 100 µl of media and incubated for 24 hr at 37°C under 5% CO2. Then, the media were changed to the non serum media containing various concentrations of rcAIM from 0 to 2.0 × 10−7 M. After 24 hr of treatment, cell numbers were counted using Cell counting kit-8 (DojinDo, Kumamoto, Japan). As shown in Fig. 2, all five histiocytic sarcoma cell lines clearly decreased in number dose-dependently. Moreover, one mammary gland tumor cell line, CHMp, and one melanoma cell line, CMM11, were also decreased by rcAIM treatment. The other cell lines, CTBp, CMM1 and TCCUB, were not so changed in their cell numbers.
Fig. 2.
Dose-responsive decrease of cell number after rcAIM treatment in various cell lines. (A) Representative cell morphology images of DH82 (histiocytic sarcoma-derived cell line) and CTBp (mammary gland tumor-derived cell line) after 24 hr of the treatment with rcAIM. DH82 cell number was reduced by rcAIM treatment, while CTBp cell number was not. Scale bars are 50 µm. (B) Dose-response curves of viabilities of canine tumor cell lines treated with rcAIM for 24 hr. 100% was determined as the cell numbers when treated without rcAIM (n=5, the mean± SE). The solid lines are histiocytic sarcoma cell lines (CHS-4, CHS-5, CHS-7, MHT-2 and DH82), and dotted lines are other tumor-originated cell lines (TCCUB: transitional cell carcinoma; CMM1 and CMM11: melanoma tumors; and CHMp and CTBp: mammary gland tumors). All the histiocytic sarcoma cell lines and two of the other cell lines showed dose-responsive cell number reduction.
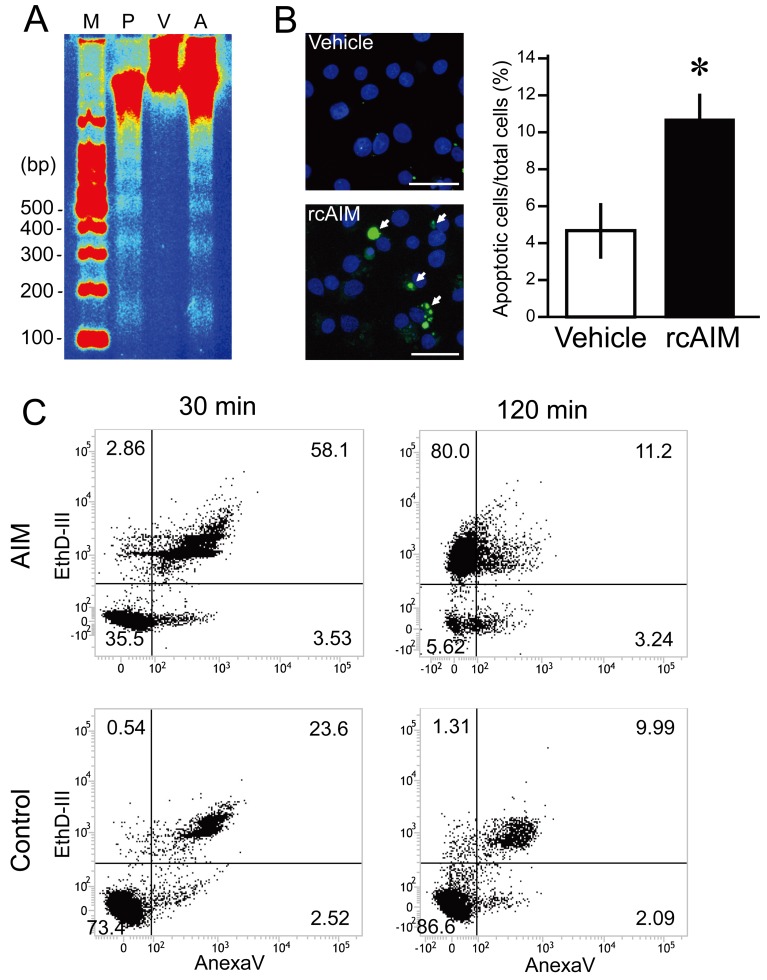
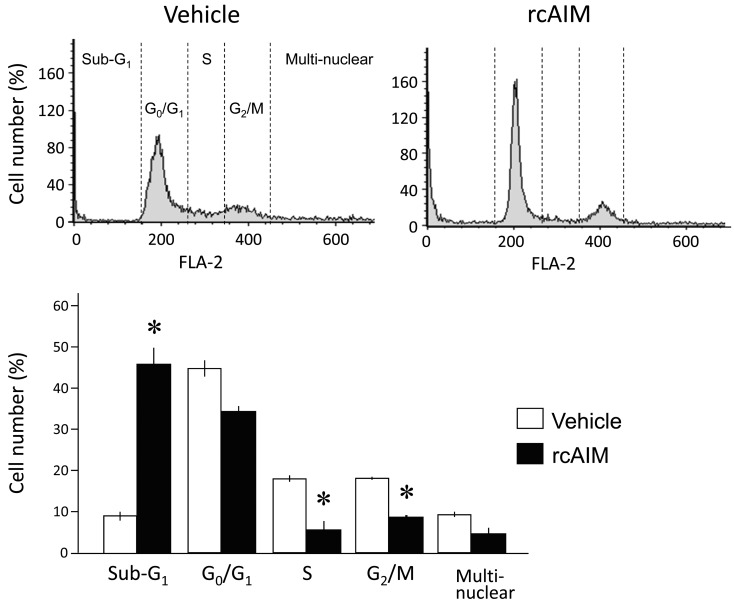
To identify the causes of cell number reduction, apoptosis analyses and cell cycle distribution were examined. For apoptosis analyses, DNA ladder detection, TUNEL staining and flow cytometry using annexin V (AnxaV) were performed. CHS-5 was seeded in 6-well plates at a density of 2.0 × 105 cells/well. After 24 hr, the medium was changed to the non-serum medium containing rcAIM (2.0 × 10−7 M). As shown in Fig. 3A, the genome DNA extracted from AIM-treated cells showed the DNA ladder pattern. TUNEL staining was performed using DeadEnd Fluorometric TUNEL System (Promega, Madison, WI, U.S.A.) according to the manufacturer’s protocol. The number of TUNEL positive cells was clearly increased 30 min after the treatment with rcAIM (Fig. 3B). This experiment was repeated three times and was demonstrated the reproducibility. For the flow cytometry, cells were analyzed using Apoptotic/Necrotic cells detection kit (PromoKine, Heiderberg, Germany) and BD FACS Verse (BD Biosciences, San Jose, CA, U.S.A.). In this method, AnxaV-positive (AnxaV (+))/ethidium homodimer III-negative (EthD-III (−)) cells indicate the early apoptotic cells, double positive cells indicate the late apoptotic cells, and AnxaV (−)/EthD-III (+) cells indicate the necrosis/end of apoptotic cells. In the group treated with AIM for 30 min, the number of double positive cells was larger compared to the other conditions (Fig. 3C). In the group treated with AIM for 120 min, the cell type of largest number of cells shifted to the AnxaV (−)/EthD-III (+) group. For cell cycle distribution analysis, flow cytometry was performed using CHS-5 cell line. After the treatment with rcAIM, cells were collected and stained with 50 µg/ml of propidium iodide solution. As shown in Fig. 4, the percentages of S and G2/M phases significantly decreased compared to the vehicle control.
Fig. 3.
Apoptotic effect of rcAIM on canine tumor cell lines. (A) Analysis of DNA fragmentation using agarose gel electrophoresis. Laddering phenomenon of fragmented DNA was observed one hr after the rcAIM treatment. M: marker, P: positive control, V: vehicle treatment, A: rcAIM treatment (2.0 × 10−7 M). (B) Apoptosis detection by TUNEL staining of CHS-5 after the treatment with rcAIM. Representative confocal microscopic images of vehicle and rcAIM treatment and the percentage of TUNEL-positive cells. Green stains indicate TUNEL signals, and blue stains indicate DAPI signals. Cells with 30 min of treatment were supposed to be at the late apoptosis stage (arrows). Scale bars are 25 µm. Asterisks mean statistical significances (P<0.05; vs control, Student’s t test). (C) AnxaV (+), EthD-III (+) cells apparently increased 30 min after AIM treatment, while only few cells showed in the vehicle control. After 120 min of AIM treatment, the most cells shifted to AnxaV (−), EthD-III (+) cells.
Fig. 4.
Cell cycle arrest caused by rcAIM treatment. Cell cycle distribution of CHS-5 was analyzed after the treatment with rcAIM (2.0 × 10−7 M). Sub-G1 significantly increased, and G1/G0 phase sharpened. Columns indicate average percentages of cell numbers at each phase (n=3, the mean± SE). Asterisks mean statistical significances (P<0.05; vs vehicle treatment, Student’s t test).
As mentioned above, canine histiocytic sarcoma is an aggressive malignant neoplasm derived from histiocytic lineage cells, such as macrophages or dendritic cells [3, 8]. While there is no direct evidence about relationship between AIM and dendritic cells in the previous reports or our data, AIM is known as an apoptosis inhibitory factor derived at least from macrophages [10, 12]. Hence, we initially hypothesized that AIM could also inhibit apoptotic reaction in histiocytic sarcoma cell lines. Surprisingly, all the histiocytic sarcoma cell lines decreased in number in a dose dependent manner when rcAIM was added to the medium. In addition, the same effect was seen with several other tumor cells, while there were few effects in the other cell lines. Indeed, there is one report about the inhibitory effect of AIM on cell viability [9]. According to this report, AIM provokes necrotic cell death, the mechanism of which involves complement-dependent cytotoxicity [9]. In this study, however, there was no complement or cytokines in the medium when AIM was treated. Moreover, apoptotic reactions and cell cycle arrest were observed in CHS-5. CHS-5 is originating from primary synovium HS [1] which has good prognosis in comparison with other non-periarticular HS [7]. To assess the mechanisms of malignant HS or clinical application for HS, it is necessary to test using the other HS cell lines as well. Even though, our findings suggest that rcAIM treatment could induce apoptosis and cell cycle arrest at least in one kind of HS cell line. It is also suggested that AIM could reduce cell number in these tumor cell lines via some different pathway (s) from the reported system.
The mechanism of this phenomenon remains unknown. As one of the candidates, we initially speculated CD36 which is a cell surface protein which can bind to AIM [8]. Since CD36 is also expressed on macrophages in dogs [15], there is a possibility that AIM can bind to macrophages via this protein. However, although CD36 was expressed in most of the histiocytic sarcoma cell lines, it was not expressed in some cell lines we tested [15]. CD36 expression levels of CHMp and CMM11 were also lower than measurement limitation (RT-PCR, data not shown), although they showed AIM sensitivity. These findings indicate that AIM could reduce the cell number via some other pathways. Further research for elucidation of AIM-mediated apoptosis and growth inhibition mechanisms is necessary.
Our findings were apparently opposite to the effect of AIM which had been reported in previous papers. There might be two reasons for that. For one reason, we used different cell types in this study. While the primary cell cultures in humans or mice were mainly used in the previous report [7, 10], the established tumor cell lines derived from dogs were used in this study. For another reason, we used different dosage of AIM. In the previous reports, they examined under the condition of quite lower concentrations of AIM (around 2.0 × 10−11 M) [7, 10]. Indeed, the lowest concentration of AIM also tended to increase cell number in the several tumor cell lines in this study. However, it is difficult to discuss which concentration is physiologically meaningful. While AIM exists at a high concentration of 3–8 µg/ml (0.6–1.6 × 10−7 M) in human [19], most AIM in plasma is reportedly complexed with IgM pentamer [6, 10]. In addition, AIM levels change depending on clinical conditions, such as atopic dermatitis, hepatitis C, liver cirrhosis and hepatocellular cancer [4, 5]. In this study, our findings just indicate that a high dose of free AIM could play a role as an anti-tumor drug to induce apoptosis and anti-proliferation of histiocytic sarcoma and several tumor cells of dogs. Since it is predicted that a high dose of AIM is needed for clinical application, it requires conventionally evaluation on normal cells in dog, including macrophage, other immune and blood cells, under condition of such dose of rcAIM in the future study.
In summary, this is the first report which demonstrates AIM as an apoptosis inducer and anti-proliferative factor in canine histiocytic sarcoma and several tumor cell lines. Further research on the mechanisms of apoptosis and growth inhibition caused by AIM in the multiple cell lines will provide a new anti-tumor approach.
Acknowledgments
This project was supported by a Grantin-Aid for Scientific Research to (B, 15H04590) from the Japan Society for the Promotion of Science to TY.
REFERENCES
- 1.Azakami D., Bonkobara M., Washizu T., Iida A., Kondo M., Kato R., Niikura Y., Iwaki S., Tamahara S., Matsuki N., Ono K.2006. Establishment and biological characterization of canine histiocytic sarcoma cell lines. J. Vet. Med. Sci. 68: 1343–1346. doi: 10.1292/jvms.68.1343 [DOI] [PubMed] [Google Scholar]
- 2.Freeman M., Ashkenas J., Rees D. J., Kingsley D. M., Copeland N. G., Jenkins N. A., Krieger M.1990. An ancient, highly conserved family of cysteine-rich protein domains revealed by cloning type I and type II murine macrophage scavenger receptors. Proc. Natl. Acad. Sci. U.S.A. 87: 8810–8814. doi: 10.1073/pnas.87.22.8810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fulmer A. K., Mauldin G. E.2007. Canine histiocytic neoplasia: an overview. Can. Vet. J. 48: 1041–1043, 1046–1050. [PMC free article] [PubMed] [Google Scholar]
- 4.Gangadharan B., Antrobus R., Dwek R. A., Zitzmann N.2007. Novel serum biomarker candidates for liver fibrosis in hepatitis C patients. Clin. Chem. 53: 1792–1799. doi: 10.1373/clinchem.2007.089144 [DOI] [PubMed] [Google Scholar]
- 5.Gray J., Chattopadhyay D., Beale G. S., Patman G. L., Miele L., King B. P., Stewart S., Hudson M., Day C. P., Manas D. M., Reeves H. L.2009. A proteomic strategy to identify novel serum biomarkers for liver cirrhosis and hepatocellular cancer in individuals with fatty liver disease. BMC Cancer 9: 271. doi: 10.1186/1471-2407-9-271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kai T., Yamazaki T., Arai S., Miyazaki T.2014. Stabilization and augmentation of circulating AIM in mice by synthesized IgM-Fc. PLOS ONE 9: e97037. doi: 10.1371/journal.pone.0097037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klahn S. L., Kitchell B. E., Dervisis N. G.2011. Evaluation and comparison of outcomes in dogs with periarticular and nonperiarticular histiocytic sarcoma. J. Am. Vet. Med. Assoc. 239: 90–96. doi: 10.2460/javma.239.1.90 [DOI] [PubMed] [Google Scholar]
- 8.Kurokawa J., Arai S., Nakashima K., Nagano H., Nishijima A., Miyata K., Ose R., Mori M., Kubota N., Kadowaki T., Oike Y., Koga H., Febbraio M., Iwanaga T., Miyazaki T.2010. Macrophage-derived AIM is endocytosed into adipocytes and decreases lipid droplets via inhibition of fatty acid synthase activity. Cell Metab. 11: 479–492. doi: 10.1016/j.cmet.2010.04.013 [DOI] [PubMed] [Google Scholar]
- 9.Maehara N., Arai S., Mori M., Iwamura Y., Kurokawa J., Kai T., Kusunoki S., Taniguchi K., Ikeda K., Ohara O., Yamamura K., Miyazaki T.2014. Circulating AIM prevents hepatocellular carcinoma through complement activation. Cell Reports 9: 61–74. doi: 10.1016/j.celrep.2014.08.058 [DOI] [PubMed] [Google Scholar]
- 10.Miyazaki T., Hirokami Y., Matsuhashi N., Takatsuka H., Naito M.1999. Increased susceptibility of thymocytes to apoptosis in mice lacking AIM, a novel murine macrophage-derived soluble factor belonging to the scavenger receptor cysteine-rich domain superfamily. J. Exp. Med. 189: 413–422. doi: 10.1084/jem.189.2.413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohashi E., Hong S. H., Takahashi T., Nakagawa T., Mochizuki M., Nishimura R., Sasak N.2001. Effect of retinoids on growth inhibition of two canine melanoma cell lines. J. Vet. Med. Sci. 63: 83–86. doi: 10.1292/jvms.63.83 [DOI] [PubMed] [Google Scholar]
- 12.Sanjurjo L., Amézaga N., Vilaplana C., Cáceres N., Marzo E., Valeri M., Cardona P. J., Sarrias M. R.2013. The scavenger protein apoptosis inhibitor of macrophages (AIM) potentiates the antimicrobial response against Mycobacterium tuberculosis by enhancing autophagy. PLOS ONE 8: e79670. doi: 10.1371/journal.pone.0079670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skorupski K. A., Clifford C. A., Paoloni M. C., Lara-Garcia A., Barber L., Kent M. S., LeBlanc A. K., Sabhlok A., Mauldin E. A., Shofer F. S., Couto C. G., Sørenmo K. U.2007. CCNU for the treatment of dogs with histiocytic sarcoma. J. Vet. Intern. Med. 21: 121–126. doi: 10.1111/j.1939-1676.2007.tb02937.x [DOI] [PubMed] [Google Scholar]
- 14.Takahashi M., Tomiyasu H., Hotta E., Asada H., Fukushima K., Kanemoto H., Fujino Y., Ohno K., Uchida K., Nakayama H., Tsujimoto H.2014. Clinical characteristics and prognostic factors in dogs with histiocytic sarcomas in Japan. J. Vet. Med. Sci. 76: 661–666. doi: 10.1292/jvms.13-0414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomura S., Uchida M., Yonezawa T., Kobayashi M., Bonkobara M., Arai S., Miyazaki T., Tamahara S., Matsuki N.2014. Molecular cloning and gene expression of canine apoptosis inhibitor of macrophage. J. Vet. Med. Sci. 76: 1641–1645. doi: 10.1292/jvms.14-0166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uyama R., Nakagawa T., Hong S. H., Mochizuki M., Nishimura R., Sasaki N.2006. Establishment of four pairs of canine mammary tumour cell lines derived from primary and metastatic origin and their E-cadherin expression. Vet. Comp. Oncol. 4: 104–113. doi: 10.1111/j.1476-5810.2006.00098.x [DOI] [PubMed] [Google Scholar]
- 17.Vail D. M., Kravis L. D., Cooley A. J., Chun R., MacEwen E. G.1997. Preclinical trial of doxorubicin entrapped in sterically stabilized liposomes in dogs with spontaneously arising malignant tumors. Cancer Chemother. Pharmacol. 39: 410–416. doi: 10.1007/s002800050591 [DOI] [PubMed] [Google Scholar]
- 18.Wellman M. L., Krakowka S., Jacobs R. M., Kociba G. J.1988. A macrophage-monocyte cell line from a dog with malignant histiocytosis. In Vitro Cell. Dev. Biol. 24: 223–229. doi: 10.1007/BF02623551 [DOI] [PubMed] [Google Scholar]
- 19.Yamazaki T., Mori M., Arai S., Tateishi R., Abe M., Ban M., Nishijima A., Maeda M., Asano T., Kai T., Izumino K., Takahashi J., Aoyama K., Harada S., Takebayashi T., Gunji T., Ohnishi S., Seto S., Yoshida Y., Hiasa Y., Koike K., Yamamura K., Inoue K., Miyazaki T.2014. Circulating AIM as an indicator of liver damage and hepatocellular carcinoma in humans. PLOS ONE 9: e109123. doi: 10.1371/journal.pone.0109123 [DOI] [PMC free article] [PubMed] [Google Scholar]