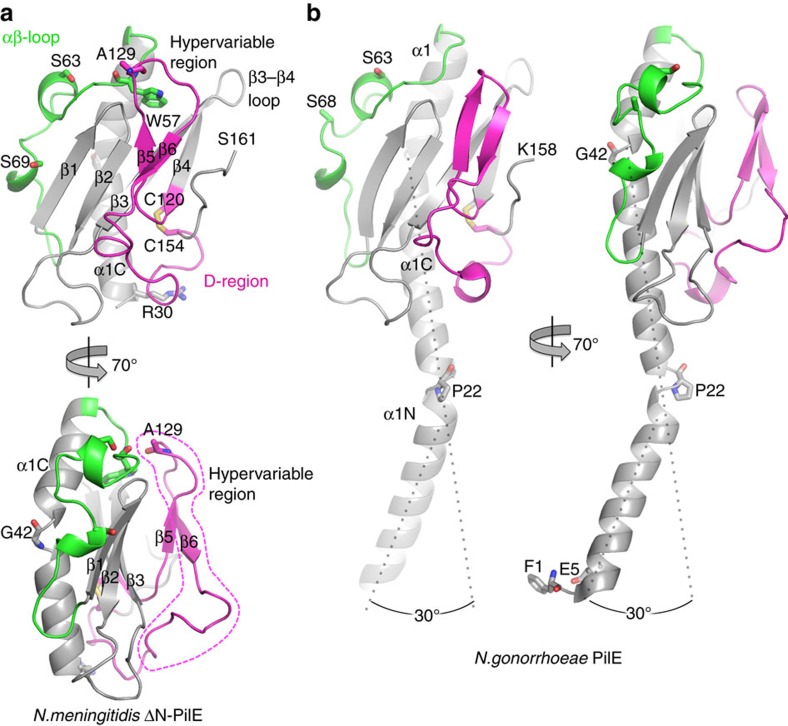
Figure 2. X-ray crystal structure of N. meningitidis ΔN-PilE and comparison with the full-length structure of Ng PilE.
Two views of (a) Nm ΔN-PilE and (b) Ng PilE pilins are shown: a ‘surface view' predicted to form the outer face of the T4P filament, and a 70° rotation. The αβ loop is shown in green and the D-region is shown in magenta, with the hypervariable region within the D-region outlined in the lower panel. Secondary structures are indicated. Nm ΔN-PilE residues Ser63 and Ser69 are post-translationally modified in the native pilin, as are the corresponding Ser63 and Ser68 shown in the full-length Ng PilE structure. The Ng PilE N-terminal α-helix, α1, is kinked at Pro22 and Gly42. The N-terminal half of α1, α1N, is absent in the Nm ΔN-PilE structure, but is identical in sequence and thus expected to share the same conformation as in Ng PilE.