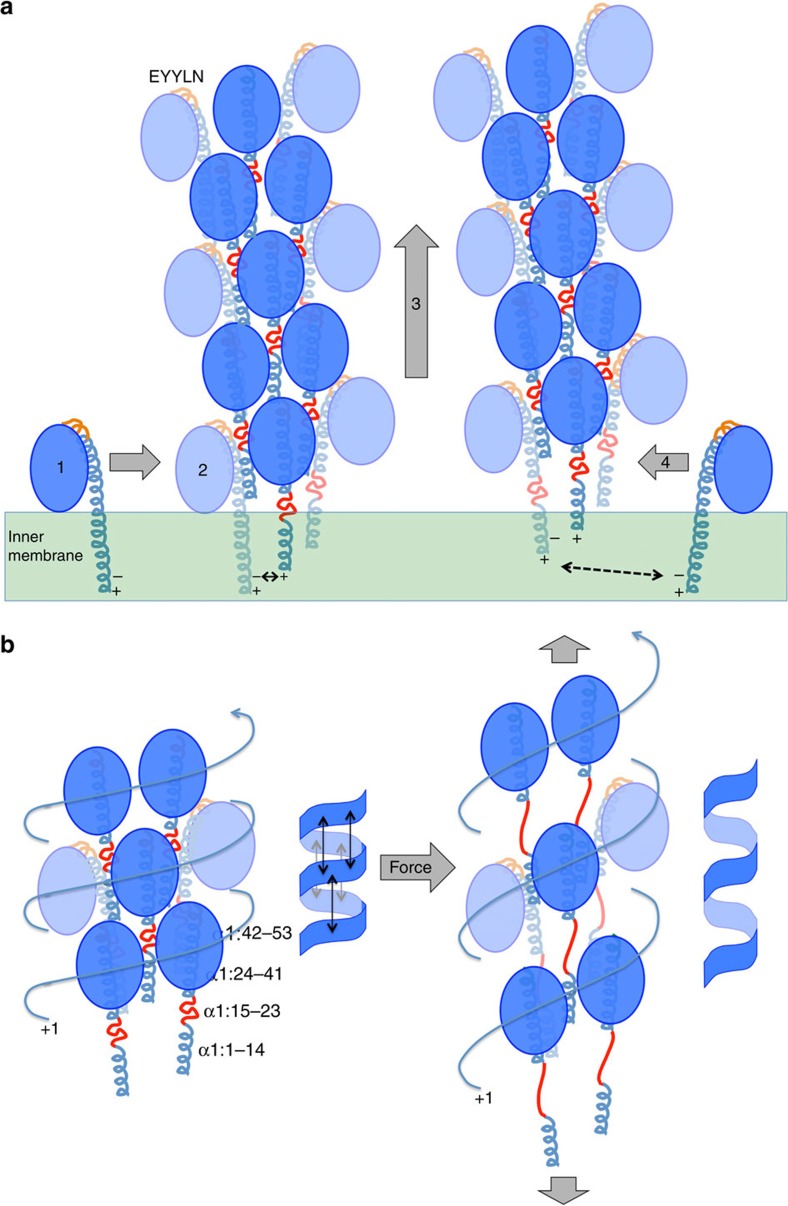
Figure 6. Models for Type IV pilus assembly and force-induced conformational change.
(a) T4P assembly model. (1) Before their incorporation into pilus filaments pilin subunits are anchored in the inner membrane with α1 in an all-helical conformation to shield backbone oxygens and nitrogens from the acyl phase of the lipid bilayer. (2) Pilin subunits dock into a growing pilus filament, attracted in part by charge complementarity between Glu5, represented by the (−) charge, and the positively charged N-terminal amine (+) on the terminal subunit in the growing pilus. (3) The pilus filament is extruded out of the membrane a short distance by the pilus assembly machinery, opening up a gap at the base of the pilus for a new subunit to dock. As the newly added pilin is extruded out of the inner membrane α1:15–23 (red) melts in order for α1:1–14 to pack into the filament core in an α-helical conformation. (4) Another subunit docks into the gap at the base of the pilus. The EYYLN epitope at the top of α1 is shown in orange. This epitope is exposed at the tip of the pilus and buried along its length. (b) Model for the force-induced conformational change of Nm and Ng T4P. Left panel: in its relaxed T4P conformation subunits are held together by lateral interactions between the globular domains along the +1-start helix and alternating α1Ns (α1:1–14) from the next turn up, as well as by axial interactions among the globular domains in consecutive turns of the +1-start helix (represented by the double-headed arrows in the schematic of the coil). The central portion of α1, α1:15–23 (red), is non-helical but compact. The EYYLN epitope (orange) is only exposed at the tip of the pilus. Right panel: under stress, the axial interactions are disrupted and α1:15–23 becomes fully extended but the lateral interactions between the globular domains and α1:1–14 are maintained for the most part, allowing a spring-like extension of the pilus to expose the EYYLN epitope all along its length.