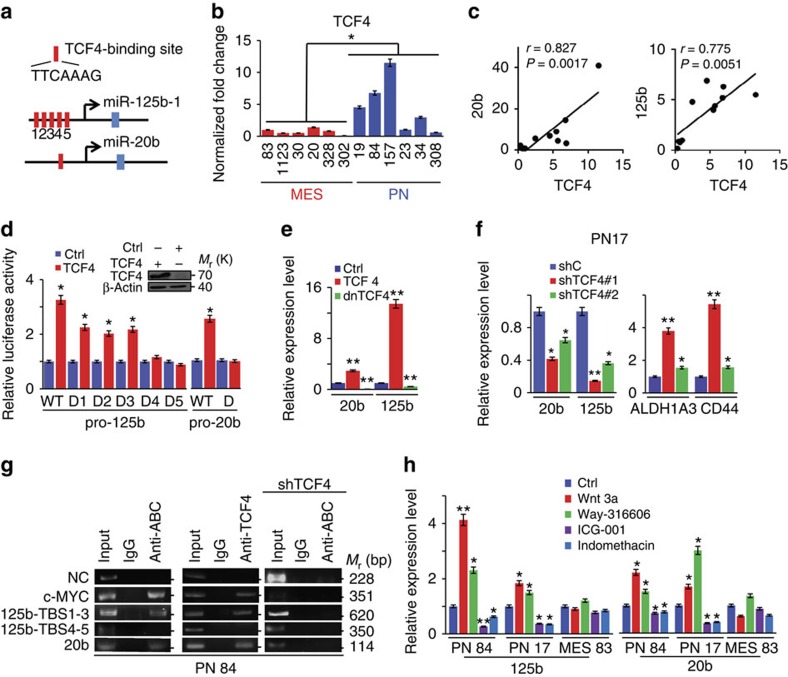
Figure 3. TCF4 induces expressions of miR-125b and miR-20b.
(a) Schematic of each TCF4-binding site (TBS) in promoter regions of miR-125b-1 and miR-20b. Blue bar: coding region for miR. Arrows: transcription start sites. (b,e–h) qRT–PCR assays. (b) TCF4 is expressed at higher levels in PN spheres compared with MES spheres. (c) Correlation plots and statistics for expressions of TCF4 and miR-125b or miR-20b in six PN and six MES spheres. (d) Dual luciferase reporter assays. The miR-125b promoter with TBS 1–3 and the miR-20b promoter with a single TBS site are critical for TCF4 induction of these miRs, respectively. D, deletion. (e) WT TCF4 induces, while a dominant negative (dn) TCF mutant suppresses expression of miR-125b and miR-20b in 293T cells. Ctrl, a control vector. (f) Knockdown of TCF4 by two separate shRNAs inhibits expressions of miR-125b and miR-20b, but increased MES-associated genes ALDH1A3 and CD44 in PN 17 spheres. shC, a scramble shRNA control. (g) Chromatin immunoprecipitation (ChIP) assays using a control IgG, anti-active β-catenin (ABC), or anti-TCF4 antibodies in PN 84 spheres with or without knockdown of TCF4. A c-MYC gene promoter containing TBSs was used as a positive control. The coding region of β-actin was used as a negative control (NC). (h) Relative expressions of miR-20b and miR-125b in PN 84 and 17, and MES 83 spheres that were separately treated with Wnt activators Wnt3a (200 ng ml−1) or WAY-316606 (20 μM), and Wnt inhibitors ICG-001 (ICG; 5 μM), indomethacin (Indo; 5 μM), or a vehicle control for 24 h. In these qRT–PCR assays, a U6 small nuclear RNA (snRNA) was used as an internal control. Data were normalized to non-treated controls. Error bars (s.d.) represent the data of triplicate samples for each cell line. *P<0.05, **P<0.01, paired two-way Student's t-test. Data are representative from three independent experiments with similar results.