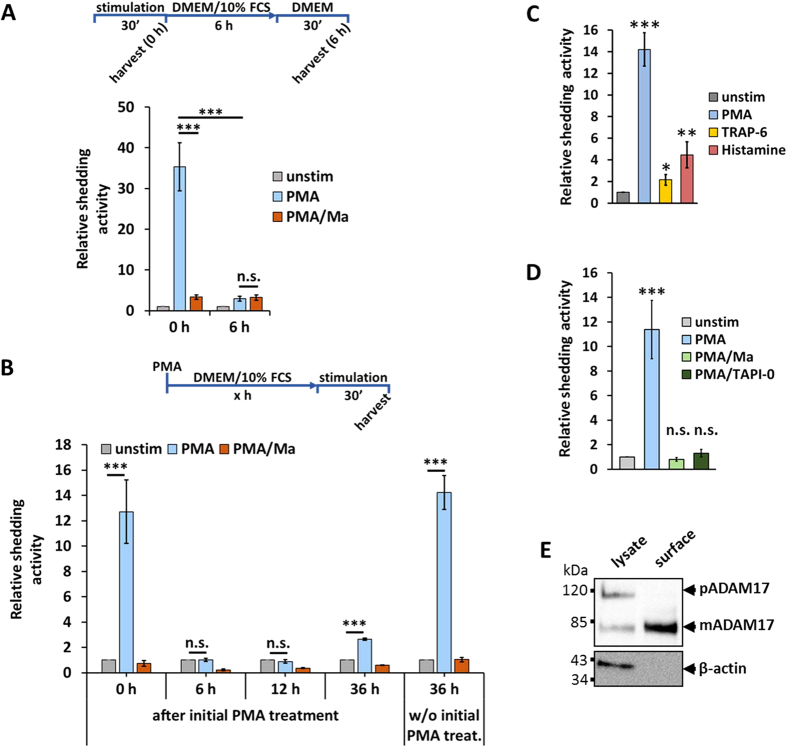
Figure 2. Effect of PMA on ADAM17-mediated shedding.
(A) HEK293 cells were transfected with AP_IL-1RII. Shedding activity was measured after a 30-minute treatment with solvent/DMSO (unstim), with 100 nM PMA or with 100 nM PMA and 10 μM marimastat (Ma). Additionally, 6 hours after the first measurement the supernatant was changed and again collected after 30 minutes without additional stimulation and shedding activity was measured again. 100 nM PMA was used as stimulator and for inhibition cells were treated with 10 μM metalloprotease inhibitor marimastat (Ma). All values were normalised to the unstimulated values. n = 4; *p < 0.05, ** p < 0.01, *** p < 0.001. (B) HEK293 cells were transfected with AP_IL-1RII. Cells were treated with 100 nM PMA and 10 μM marimastat to block the loss of substrate. After indicated time, shedding activity was measured after a 30-minute treatment with solvent/DMSO (unstim), with 100 nM PMA or with 100 nM PMA and 10 μM marimastat (Ma). All values were normalised to the unstimulated values. n = 4; *p < 0.05, **p < 0.01, ***p < 0.001. (C) ADAM17 activity comparison of the different stimulators normalised to the unstimulated (unstim) samples. n = 3; *p < 0.05, **p < 0.01, ***p < 0.001. (D) HEK293 cells were transfected with AP_IL-1RII. Shedding activity was measured after a 30-minute treatment with solvent/DMSO (unstim), with PMA or with PMA and an inhibitor. 100 nM PMA was used as stimulator. For inhibition, cells were treated with following compounds: 10 μM membrane-permeable metalloprotease inhibitor marimastat (Ma) or 15 μM non-membrane permeable metalloprotease inhibitor TAPI-0. All values were normalised to the unstimulated values. n = 4; *p < 0.05, **p < 0.01, ***p < 0.001. (E) Western blot of HEK293 lysate and HEK293 cell-surface proteins (surface biotinylation). n = 3.