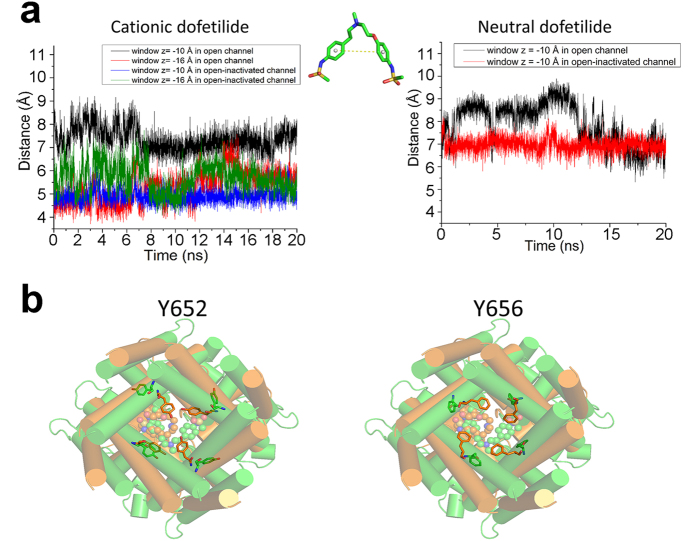
Figure 4. Conformation dynamics of bound dofetilide and coordinating residues in the hERG channel from free energy simulations.
(a) Left: distance of benzene rings of cationic dofetilide in the inner binding site for the open (black) and open-inactivated (blue) channel and in the outer binding site for the open (red) and open-inactivated (green) channel. Middle: sketch map of the distance between two benzene rings. Right: distance of benzene rings of neutral dofetilide in the inner binding site for the open (black) and open-inactivated (red) channel. (b) Conformational changes of Y652 and F656 in the open (green) and open-inactivated (orange) channel.