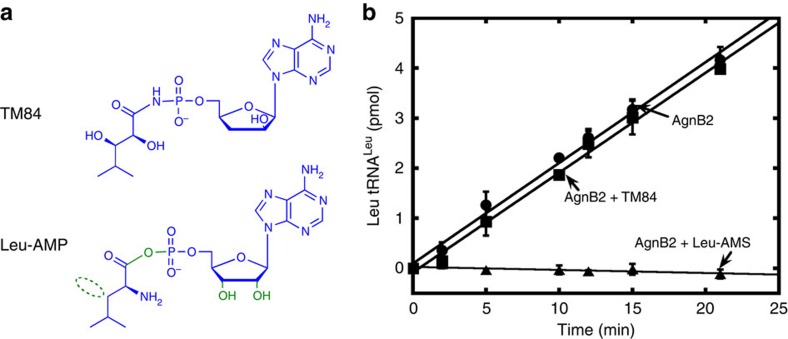
Figure 2. AgnB2 discriminates between a stable Leu-AMP analogue and TM84.
(a) Chemical structures of Leu-AMP and TM84 with differences highlighted in green. A non-hydrolysable adenylate analogue called Leu-AMS, which contains a N-sulfamoyl linkage in place of the phosphoanhydride of Leu-AMP, was used in biophysical and crystallography experiments. (b) Effect of TM84 and Leu-AMS on the aminoacylation reaction of wt AgnB2. Aminoacylation reactions were carried out using 2 nM wt AgnB2 only (●) and wt AgnB2 in the presence of 1 μM TM84 (■) and 20 nM Leu-AMS (▲) at 28 °C, pH 7.4 and initiated using 1 mM ATP. Error bars represent s.d. (n=3).