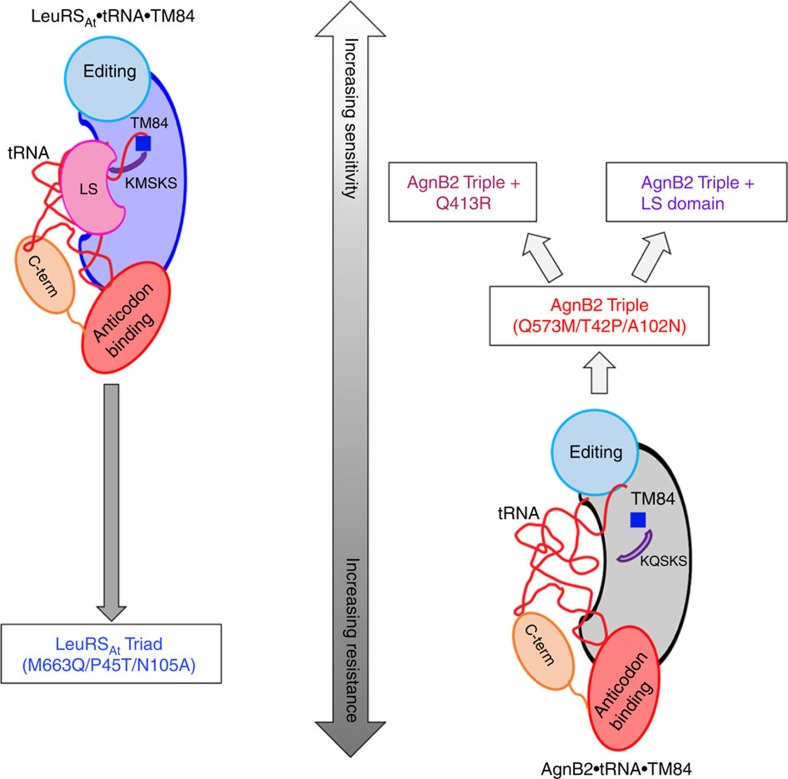
Figure 8. Model illustrating determinants of TM84 sensitivity and resistance in LeuRSAt and AgnB2.
The ternary complex of LeuRSAt forms a tight binding complex with the CCA 3′-end of the tRNA and TM84. Upon mutation of three critical residues, P45, M633 and N105, a TM84 resistant mutant (LeuRSAt Triad) is obtained. AgnB2 on the other hand forms a weak ternary complex with tRNA and TM84, thereby explaining its high level of resistance towards TM84. Mutagenesis of the corresponding residues in AgnB2 with the residues in LeuRSAt results in the AgnB2 Triple mutant that has reduced levels of AgnB2 resistance. Upon introduction of Q413R mutation or addition of a LS-domain to the AgnB2 triple mutant, increased sensitivity towards TM84 is achieved. Thus these residues along with the LS domain may be the key determinants of sensitivity or resistance in LeuRSAt and AgnB2.