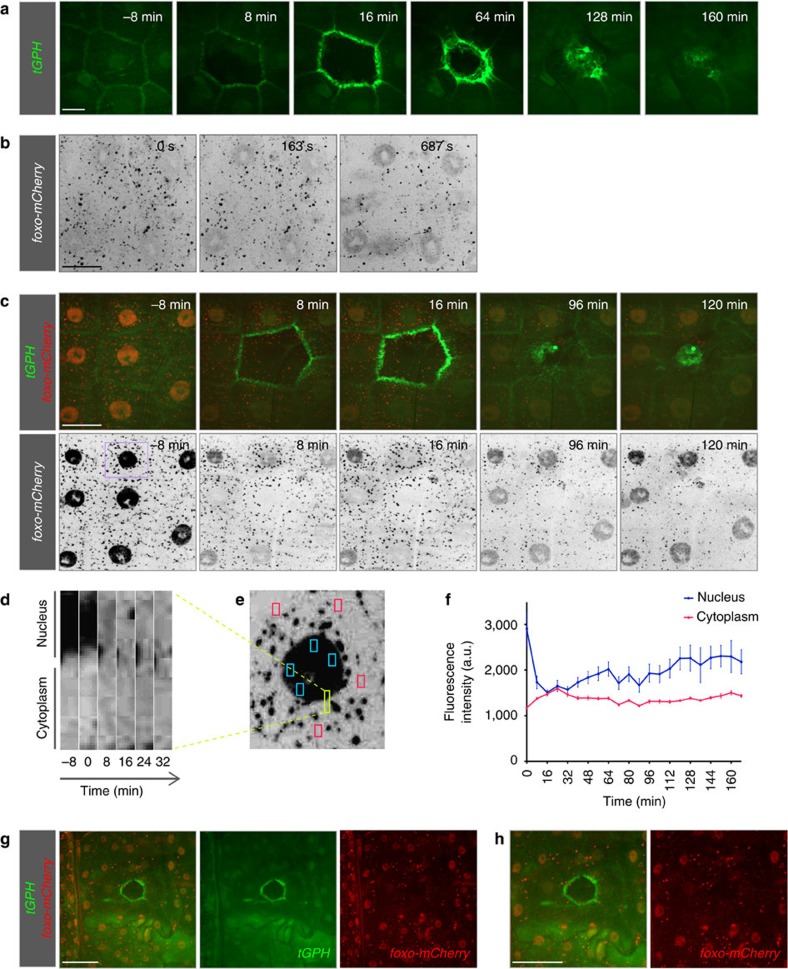
Figure 2. Insulin signalling during wound healing.
(a) Distribution of tGPH, a reporter for PIP3. tGPH, the PH-domain of GRP1 coupled to GFP, is expressed under the tubulin promoter in all tissues. At 7±1 min, tGPH becomes polarized from an initially ubiquitous subcellular distribution in cells surrounding the wound. (b–h) Subcellular distribution of FOXO before wounding and during wound healing in L3 larvae in which mCherry was inserted into the foxo locus, generating a FOXO-mCherry gene product expressed under the endogenous foxo promoter. (b) Effect of mounting and imaging on FOXO distribution. When larvae were first mounted for imaging, FOXO-mCherry (grey) was ubiquitously distributed in the cytoplasm and in the nuclei of epidermal cells. Soon after mounting under the spinning disk confocal microscope and laser illumination (561 nm), FOXO-mCherry became enriched in the nuclei. The intense punctate pattern is seen with all mCherry constructs we have used in this study (see also Fig. 1e, for example). (c) Time-course of redistribution of epidermal FOXO-mCherry (red in upper panel and grey in lower panel) after wounding; the larvae also expressed tGPH (green). In cells directly surrounding the wound, nuclear FOXO began to translocate to the cytoplasm within 7±1 min of wounding. By 16±1 min nuclear FOXO had reached its minimum. (d) Kymograph of FOXO distribution in a region of a cell bordering the wound (yellow marked area in e). (e) Overview of the cell for which the analyses are shown in d and f with the analysed areas marked by coloured boxes. (f) Change in fluorescence intensity in four areas of the cytoplasm (red boxes in d) and nucleus (blue boxes in d) plotted against time. Mean±s.e.m. (g) Lower magnification view of subcellular distribution of FOXO-mCherry (red) 10 min after wounding showing cells further beyond the wound edge. More distant cells did not respond to wounding and FOXO-mCherry remained nuclear. Scale bars, (a) 20 μm, (b,c) 30 μm and (g,h) 60 μm. L3: third instar larva.