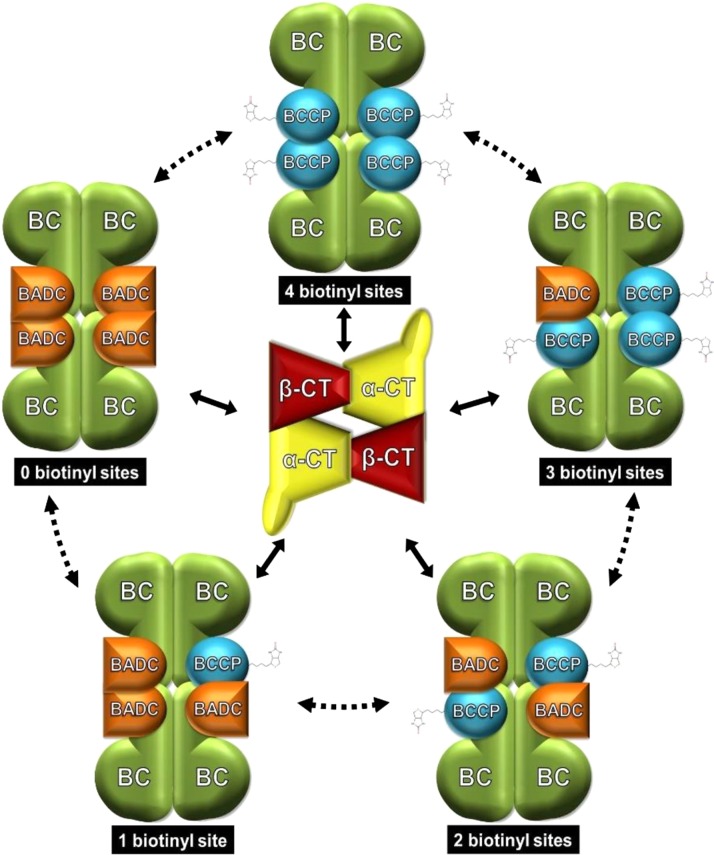
Figure 10.
Model of BADC Competitive Inhibition of hetACCase.
Schematic illustrating the proposed mechanism of BADC inhibition. The BC/BCCP subcomplex design was made based on the crystal structure in E. coli (Broussard et al., 2013), consisting of two dimers of BC and four BCCP proteins. The BADC proteins compete with BCCP for binding to BC. Binding of BADC prevents binding of the essential BCCP subunit. The pool of BC/BCCP and BC/BCCP/BADC subcomplexes then compete for interaction with the CT subcomplex (design based on crystal structure in E. coli; Bilder et al., 2006), leading to variable reductions in ACCase activity. While a transient association of the two ACCase half reactions is known, it is unclear whether BADC can displace BCCP from an assembled BC/BCCP subcomplex, hence the dashed arrows.