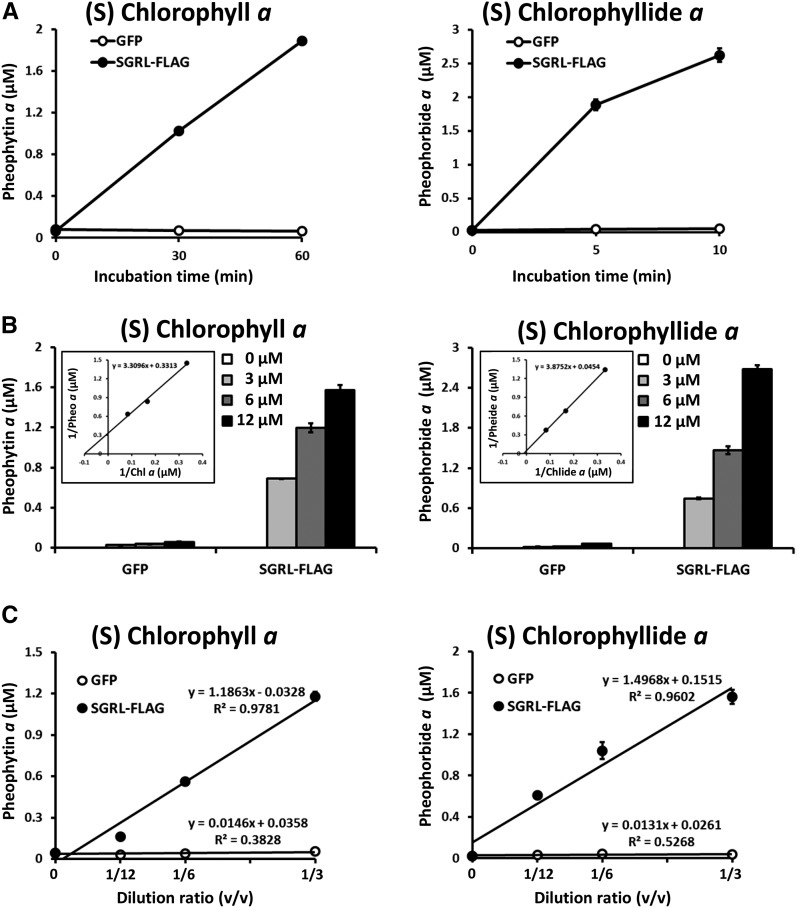
Figure 4.
Biochemical Analysis of SGRL.
(A) Time-dependent formation of Mg-free chlorophyll derivatives by SGRL-FLAG. Chlorophyll a or chlorophyllide a were incubated with recombinant GFP (open circles) and SGRL-FLAG (closed circles) for up to 60 min or 10 min at 25°C, respectively. Recombinant proteins were prepared by a wheat germ protein expression system and diluted 3-fold with the reaction buffer without purification. GFP was used as a negative control because it has similar molecular weight as SGR. The concentration of substrates was 6 µM. After incubation, the level of pheophytin a and pheophorbide a was determined using HPLC (n = 3 ±sd).
(B) Kinetic analysis of Mg-dechelating of SGRL-FLAG. Various concentrations of chlorophyll a or chlorophyllide a were incubated with recombinant GFP and SGRL-FLAG for 30 or 5 min at 25°C, respectively. Recombinant proteins were prepared by a wheat germ protein expression system and diluted 3-fold with the reaction buffer without purification. GFP was used as a negative control because it has similar molecular weight as SGR. After incubation, the level of pheophytin a and pheophorbide a were determined using HPLC (n = 3 ±sd). The inset shows Lineweaver-Burk plot of kinetic data of Mg-dechelating of SGRL-FLAG.
(C) SGRL-FLAG concentration-dependent formation of Mg-free chlorophyll derivatives. Chlorophyll a or chlorophyllide a were incubated with various concentrations of recombinant GFP (open circles) and SGRL-FLAG (closed circles) for 30 or 5 min at 25°C, respectively. Translation solutions containing expressed GFP and SGRL-FLAG were diluted 3, 6, or 12 times in 50 μL of reaction buffer. GFP was used as a negative control because it has similar molecular weight as SGR. The concentration of substrates was 6 µM. After incubation, the levels of pheophytin a and pheophorbide a were determined using HPLC (n = 3 ±sd).