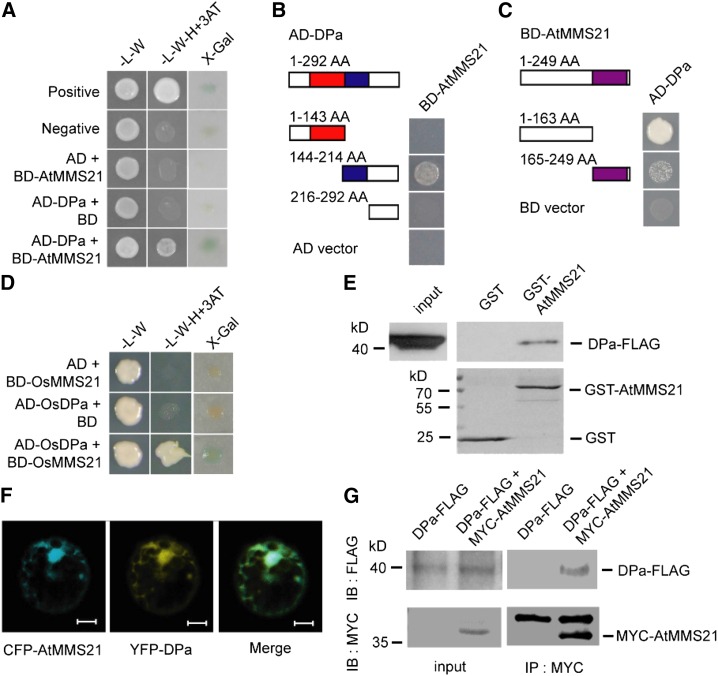
Figure 2.
DPa Interacts with AtMMS21.
(A) The interaction between DPa and AtMMS21 was confirmed in a yeast two-hybrid assay.
(B) Identification of the domain on DPa required for its interaction with AtMMS21 by yeast two-hybrid analysis. The DNA binding domain (red) and dimerization domain (blue) of DPa and the SP-RING domain (purple) of AtMMS21 are indicated.
(C) Identification of the domain on AtMMS21 required for its interaction with DPa by yeast two-hybrid analysis.
(D) The interaction between rice DP and MMS21 in a yeast two-hybrid assay.
(E) The interaction between DPa and AtMMS21 in an in vitro pull-down assay. The DPa-FLAG proteins were incubated with immobilized GST or GST-AtMMS21, and proteins immunoprecipitated with glutathione sepharose were detected using anti-FLAG antibody. The amounts of GST and GST-AtMMS21 are shown in the bottom panel.
(F) The subcellular colocalization of DPa and AtMMS21. CFP-AtMMS21 and YFP-DPa were coexpressed in protoplasts. After 48 h, the CFP and YFP signals were detected and merged. Bar = 10 µm.
(G) The interaction between DPa and AtMMS21 in an in vivo coimmunoprecipitation assay. Total protein extracts from transgenic plants carrying 35S:DPa-FLAG alone or both 35S:DPa-FLAG and 35S:MYC-AtMMS21 were immunoprecipitated with the immobilized anti-MYC antibody. The proteins from crude lysates (left) and immunoprecipitated proteins (right) were detected using anti-FLAG or anti-MYC antibody.