Abstract
Sustained and controlled pellets are considered as one of the ideal dosage forms. Due to the large coverage area of pellets, loaded drugs can be absorbed completely in the body and bioavailability is improved correspondingly. Coated pellets-containing tablet is a special oral formulation consisting of various pellets with different release rate. Desired rate of drug release rate can be achieved by adjusting the proportion of pellets. However, this formulation faces strict requirements in the process of preparation. Several factors will influence release behavior of tablets, including pellet cores, coating, and tabletting. Therefore, these factors will be investigated sufficiently in this review to provide valuable information for manufacturing process.
Keywords: Tablet, Pellet, Quality factors, Formulation factors, Technological factors
1. Introduction
Orally sustained and controlled drug delivery systems are usually used to improve release behavior of drugs to meet different conditions in the body, which includes single unit dosage forms (SUF) and multiple unit dosage forms (MUFs) (Abdul et al., 2010). The latter consists of pellets, granules, microparticles and minitablets. Compared with SUF, MUFs present several advantages (Liu et al., 2012): (i) The multiparticulates spread uniformly throughout the gastrointestinal tract (GIT), which can reduce local irritation of active ingredient, enhance drug absorption and lower the fluctuation of peak plasma. (ii) MUFs possess constant transit time in the GIT, which can avoid dose bumping and improve safety. (iii) The defect of individual unit has no serious effect on efficacy. (iv) Inter- and intra-individual variations in the bioavailability caused for instance by food effects can also be reduced.
Pellets are a class of globular entity consisting drugs and excipients, which normally are no more than 2.5 mm in diameter. Coated pellets have effects of sustained release and masking taste. Tablets consisting coated pellets can contain incompatible drugs or drugs with different release rates. In contrast with pellet-containing capsules, tablets are more tamper resistant, easier to swallow and of lower cost in industrial production. With the application of coated pellets-containing tablets, tablets will be completely disintegrated into pellets and drug can be subsequently released from the pellets (Table 1). In fact, the MUF should not fuse into the non-disintegrating matrix during compaction and then disintegrate rapidly into the individual pellets in GIT. Compaction process has no effect on the release characteristics.
Table 1.
Overview of marketed tablets containing coated pellets.
| Trade name | API | Therapeutic role | Formulation | Manufacturer |
|---|---|---|---|---|
| Betaloc ZOK | Metroprolol succinate | Hypertension | Multi-unites sustained pellet-containing tablet | AZN |
| Harnal D | Tamsulosin Hydrochloride | Hyperplasia of prostate | Multi-unites sustained pellet-containing disintegrating tablet | Astellas |
| Losec MUPS | Omeprazole Magnesium | Peptic ulcer | Multi-unites enteric pellet-containing tablet | AZN |
| Nexium | Esomeprazole magnesium | Peptic ulcer | Multi-unites enteric pellet-containing tablet | AZN |
| Prevacid | Lansoprazole | Peptic ulcer | Multi-unites enteric pellet-containing disintegrating tablet | Takeda |
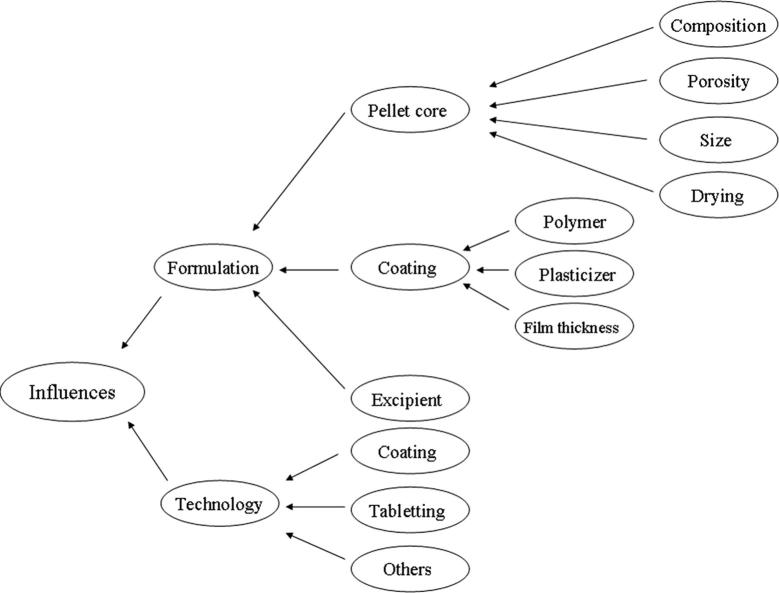
Compared with the preparation of common tablets, the technologies of pellet-containing tablets are more complex. At present, available tablets in the market mainly include proton pump inhibitors and some drug with narrow therapeutic window (Table 1). The key point in the preparation of coated pellet-containing tablet is to ensure the integrity of the coating film, which must be able to withstand the compression force. Many studies (Altaf et al., 1998, Miller et al., 1999) showed that coating films usually suffer from damages in the compression process, which will influence drug release behavior. Current researches mainly focus on the optimization for type and diameter of pellet cores, type and dosage of coating polymers and plasticizer, buffer excipients, as well as technological parameters (Fig. 1). All of these will be discussed specifically in this review and put forward corresponding solutions.
Figure 1.
Influence factors on quality of pellet-containing tablets.
2. Formulation factor
2.1. Pellet core
Core features of coated pellets are essential to the tabletting of coated pellets. Cores, with good elasticity and toughness, ought to meet the requirements of deformation during tabletting, which can reduce undesirable effects following elastic recovery on coating films and ensure intact coating films. Determinant factors of tensile strength contain core composition, size, porosity, and so on. Coated cores with good tensile strength can withstand pressure in the tabletting process and maintain intact coating film.
2.1.1. Core composition
Pellets itself must have certain tensile strength, and thus maintain release behavior of pellets following tabletting. The first issue to be considered is the composition of pellets. Components of pellets include diluents and adhesive, such as lactose, starch, microcrystalline cellulose (MCC), hydroxymethyl cellulose, polyvinyl alcohol, Polyvinylpyrrolidone (PVP), and hydroxypropyl methyl cellulose (HPMC).
Due to the good rheological properties that enhance the plasticity of other excipients and produce bonding effects, MCC is called balling promoter and applied widely. The mechanism of tabletting with MCC-containing pellets was studied by Johansson et al. (1995). The result showed that pellets presented permanent deformation not fragmentation in the tabletting process. The increase in deformation degree of pellets would decrease distance between pellets, which ultimately strengthened binding force among pellets and increased hardness of tablets in the meantime. For the core materials with good rheological properties, the tabletting force can be increase appropriately. In addition, some excipients can further improve compressibility of MCC-containing pellets. Pellets formed from MCC with other excipients were more resistant to compression than those formed from MCC alone, such as lactose/MCC, dibasic calcium phosphate/MCC. A soft material (polyethylene glycol) can also be used to modulate compression behavior and compressibility of the pellets (Nicklasson and Alderborn, 1999). This change possibly is due to the pellets composed of blended excipients behaved differently during deformation.
However, for insoluble drugs, drugs of chemical incompatibility with MCC, and some other easily adsorbed on MCC fibers, MCC will probably retard drug release to some extent. At this time, MCC is inappropriate to be used to make pellet cores. Bornhöft et al. (2005) found that к-carrageenan was a very promising substitute for MCC in pelletization. Compared with MCC pellets, the preparation of к-carrageenan pellets needed more water, and presented more robust with respect to fluctuations in water content. Meantime, systematic investigations were conducted to evaluate effects of other ingredients. Four drugs (acetaminophen, mesalamine, theophylline and hydrochlorothiazide) and four fillers (lactose, mannitol, maize starch and dicalciumphosphate dihydrate) were selected in the experiment (Thommes and Kleinebudde, 2006, Thommes and Kleinebudde, 2006). The result showed that all pellets have good shape and size, which indicated that к-carrageenan was a suitable pelletization. Poorly soluble drugs loaded in к-carrageenan pellets by wet extrusion–spheronization presented better release behavior than MCC pellets. This was attributed to the time poorly soluble drugs releasing from MCC pellets exceeding the gastro-intestinal passage time. Ghanam and Kleinebudde (2011)) developed bisacodyl sustained pellet-containing tablets. Pellets were prepared by bisacodyl and к-carrageenan or MCC, and coated with a mixture of Kollicoat® MAE 30 DP and Eudragit® NE 30 D. In vitro dissolution test showed that к-carrageenan pellet tablets released over 80%, but MCC pellet tablets were only about 20–24%.
2.1.2. Porosity
The pellet porosity determined the degree of their deformation, and would subsequently influence compression of pellets and release behavior of drugs. Johansson and Alderborn (1996) investigated the degree of deformation and densification of MCC-containing pellets. The results showed that the low porosity pellets presented only limited local permanent deformation and no changes in porosity during compression, but high porosity pellets with the same particle size appeared a big change in shape and porosity. Tunón et al. (2003) investigated the deformation degree and release behavior of various reservoir pellets with different porosity after compression, and found that the deformation degree of pellets followed tabletting was directly related to the change of amount of drug release. Pellets with high porosity appeared high degree of deformation and densification, but release behavior of drug was hardly influenced, which indicated no destruction on coating film of pellets. Scanning electron microscopy also presented that coating film did not tend to become convoluted and still cover firmly on the pellet surfaces. This could be attributed to the increase of cushion space of coating film when pellets with high porosity were compressed, which reduced destructive effects from compaction and change of permeability of pellets. In contrast, the cushion space of pellets with low porosity diminished and the large proportion of pellets was in contact with the punches and die during compaction. Consequently, the risks of damage on coating films were enhanced. Compaction of pellets with high porosity would considerably affect the degree of densification and deformation, but slightly effect on drug release. For low porosity pellets, the influence of compaction was obvious, whereas degree of densification and deformation was slight. Accordingly, pellets with high porosity are more suitable to be made into tablets to maintain release behavior. After regulating porosity of pellet cores, compaction technologies can be optimized to achieve ideal drug release.
2.1.3. Particle size
Different diameters of pellet cores will directly influence compressibility of tabletting excipients, and subsequently have effects on compression performance of pellets and drug release. Johansson et al. (1998) developed two MCC-containing pellets with different diameter (i.e. 425–500 μm and 1250–1400 μm) by extrusion–spheronization. Meantime, the influences of tabletting of coated pellets dominating mechanisms of compression were investigated. The porosity and tensile strength of tablets were determined, and the results showed that mechanisms of compression for the pellets were deformation and densification. The original size of pellets did not affect porosity changes of tablets, but the degree of deformation of individual pellets was related with core sizes, i.e. degree of deformation enhanced with increasing size. Dashevsky et al. (2004) found that smaller pellets, at the same weight, presented the higher surface area, and thus had a thinner coating and tended to the rupture of coating films. The release rate of drugs from coated pellets slowed down with the increase of core size and coating level. The propranolol HCl release from Kollicoat® SR 30 D coated pellets decreased with increasing pellets size.
2.1.4. Core drying
Drying methods on pellet cores can affect porosity of pellets and subsequently influence compression performance of pellets. Four different techniques (Bashaiwoldu et al., 2004), including freeze-drying, fluid-bed drying, hot air oven drying and desiccation with silica-gel, were compared and summarized. According to the different rate of moisture removal, heating method and mass transfer, the different drying techniques give rise to pellets of different structural and mechanical properties. The results showed that deformability was dependent with drying techniques, and could be sorted from low to high, i.e. freeze-drying, fluid-bed drying, hot air oven drying and desiccation with silica-gel. The deformability of pellets was positively related with porosity, and pellets tended to deform with increasing porosity. Moreover, drying rate had effect on the deformability and compressibility of pellets, and deformability of pellets increased along with the enhancement of drying rate.
2.2. Coating
Polymeric coatings are applied to meet various purposes in pharmaceutical field. They can control drug release from oral dosage forms, improve the chemical stability of drugs by setting up a physical barrier to the influence of external environmental, and mask repulsive taste and odor (Ensslin et al., 2009, Goole et al., 2008, Ye et al., 2007). Several problems related with polymeric materials (Technological factors will be explained blow) maybe occur in the coating process. With increasing temperature, mobility of polymer chains increases, boundary between polymer particles eliminates, polymer particles bond mutually, and coating membranes take shape ultimately after mechanical deformation. But in the meantime, incomplete fusion between colloidal particles perhaps happens in the coating process. Shrinkage of film usually produces residual cohesion during solvent evaporation. Thermal expansion properties between coating film and substrate exist differences. All these elements can contribute to the cracks or defects of coating films. Moreover, shape and density of coated pellets change sharply during tabletting, as well as friction and collision between pellets and die possibly damage polymeric film. These two crucial reasons will deprive pellets of controlled release performance. In consequence, before compression of coated pellets, different mechanical properties that coating films presented during receiving different types of stress should be investigated completely.
2.2.1. Coating polymer
Polymers currently used in the coating fall into two broad groups: cellulosic and acrylic polymers. Cellulosic polymer, such as ethylcellulose, is used for extended-release. The acrylic polymers are marketed under the trade names of Eudragit® or Kollicoat®, which mainly are used to control drug release and mask taste. These two polymers can be formulated as aqueous colloidal dispersions and organic solutions.
Due to the low puncture strength and elongation (<5%) and weak mechanical properties, EC used for coating film tends to be damaged and then loses the function of sustained-release (Bodmeier and Paeratakul, 1994). Mechanical properties of EC were hardly strengthened by adding plasticizer, nor curing of the pseudo-latex-cast ethylcellulose films worked. Hosseini et al. (2013) tried to layer tabletting excipients (e.g., MCC, lactose or sorbitol) onto the ethylcellulose-coated pellets to form a cushion layer to protect the integrity of the brittle ethyl cellulose coating during compression. However, the result showed that tabletting excipients are still insufficient to maintain the integrity of coating film. Only incorporating glidant (e.g., magnesium stearate or aerosil) into the cushion layer or between the cushion layer and the ethyl cellulose coating, the compression effect on drug release can be reduced. Nevertheless, craft process became complex. Elongation of EC film can be improved by combining EC aqueous dispersion with Acryl-EZE®. Acryl-EZE® is a fully formulated, dry enteric acrylic coating system dispersible in water, for the application of an enteric film coating to multi-particulate solid dosage forms. Li et al. (2012) prepared aspirin pellets coated with Surelease® (aqueous ethylcellulose dispersion) and Acryl-EZE®. In vitro tests showed 30% aspirin released from tablets at the first two hours, and the rest released in the later 10 h. Weibull curve fitting equations indicated that drug release from pellet-containing tablets met first-order rate process.
Compared with EC films, acrylic polymers are more suitable for coating of pellets intended to be compressed into tablets (Bodmeier and Paeratakul, 1994). Eudragit® NE 30 D is the aqueous dispersion of a neutral copolymer based on ethyl acrylate and methyl methacrylate. This dispersion was highly flexible and suitable for matrix structure. Due to the high elongation at break of approximately 600%, Eudragit® NE30D does not need to combine with addition of plasticizers and was flexible enough to withstand deformation forces during tabletting. Eudragit® RS and Eudragit® RL 30D showed insufficient elongation, but they also can be used for tabletting after adding plasticizers. Abbaspour et al. (2008) developed ibuprofen disintegrating sustained-release tablets comprising coated pellets. Tablets were designed to disintegrate into sustained-release pellets after oral ingestion. In order to maintain the integrity of the pellet films, coated membrane materials contained Eudragit® RS 30D and RL 30D in 4:1 ratio and different levels of triethyl citrate. Mechanical tests showed that the coated pellets had no significant effect on yield point and elastic modulus of the pellets. Scanning electron microscope graphs and in vitro dissolution test showed no apparent damage to the coated pellets after compaction process.
Eudragit® L 30D55 is the aqueous dispersion of anionic polymers with methacrylic acid as a functional group and a milky white liquid of low viscosity. Due to the strong interchain hydrogen bonding caused by the impacts of carboxyl groups, Eudragit® L 30D55 resulted in weak and brittle films. Pellets coated with Eudragit® L 30D55 alone are easy to appear with cracks in the range of 5–50 μm after compression, and cannot meet the requirements of enteric formulations and sustained-release. Eudragit® L 30D55 can be blended with Eudragit® NE 30D with good flexibility, and this mixed coating films at a certain proportion can maintain the integrity of pellets. When the ratio of Eudragit® NE 30D in blends is more than 10%, film strength of pellets can be enhanced significantly and more suitable for tabletting, but too much dosage will retard drug release in the intestinal tract. EI-Malah and Nazzal (2008) tried to evaluate the mechanical and thermal properties of films prepared from Eudragit® NE 30D and L 30D-55 blends. With the increase of Eudragit® NE 30D concentrations, miscibility, softness, and decreased stiffness of the films enhanced correspondingly. Cast films were evaluated by texture analysis and differential scanning calorimetry. The mixture proportions of Eudragit® NE 30D and L 30D-55 respectively are 50:50, 67:33, 75:25 and 80:20, which were evaluated by texture analysis and differential scanning calorimetry. The data obtained showed that polymeric blends can be permitted as coating materials. When the concentration of Eudragit® NE 30D is at least 80%, miscible and homogenous blends could be obtained, which influenced drug release. The lag time and drug release rate were controlled by the theoretical weight gain of the beads and the concentration of Eudragit® NE 30D. The blend at 80:20 ratio retarded drug release by approximately 7 h, but the blend at 80:20 ratio just only delayed drug release by only 3.5 h. This was attributed to the homogeneous mixture of the two polymers. Apart from screening coating materials and adding plasticizers, additive protection can be employed to withstand the compression force during tabletting. Mesalamine sustained-release pellets coated with Eudragit® S100 were compressed into tablets and then coated with additional polymeric films (Eudragit® L 100-55) for colon-specific drug delivery (Bendas et al., 2010).
Kollicoat® belongs to Polyvinylacetate copolymer colloidal substances developed by BASF. Glass transition temperature of Kollicoat® is only in the range of 5–18 °C. Due to the certain viscosity, antiadhesion agents will be added during coating or storing. Dashevsky et al. (2004) compared three types of membrane materials, Kollicoat® SR 30 D, Aquacoat® ECD 30 and blends of Kollicoat® MAE 30 DP and Kollicoat® EMM 30 D. The results showed that the blends of Kollicoat® MAE 30 DP and EMM 30 D maintain sufficient enteric properties of pellets. Kollicoat® SR 30 D combined with 10% w/w triethyl citrate could be used as film material. While pellets coated with Aquacoat® ECD 30 were easy to rupture during compression.
2.2.2. Plasticizer
Plasticizers work by embedding themselves between the chains of polymers, spacing them apart and thus significantly lowering the glass transition temperature for the plastic. Concentration of plasticizer in the coating solution depends on several factors, including polymeric properties, usage, and other additives.
Given special requirements for the coating films of pellets during compression, types and amount of plasticizer must be investigated in-depthly. The frequently used plasticizers include triethyl citrate (TEC), dibutyl sebacate (DBS), propylene glycol (PPG), polyethylene glycol (PEG). Sawicki and Lunio (2005) designed verapamil hydrochloride floating pellets coated with Kollicoat® SR30D. Three plasticizers were examined PPG, TEC and DBS (all at concentration of 10%). Due to the solubility of TEC and DBS being less than PPG, drug release was retarded and in vitro release presented significant differences. This was attributed to the different properties of drugs, hydrophilic or hydrophobic plasticizers present variant effects. Optimal plasticizers should present compatibility with polymers, solubilize polymers and reduce Tg of polymers to the greatest extent.
In addition, moisture is also essential for the successful compression of coated pellets. Rujivipat and Bodmeier (2012) investigated how important moisture play role in the coating films properties. Coated pellets were stored at different humidity at room temperature for 1 month, including 52%, 75%, 84%, 95% and 100% RH. Tg of coating film ultimately lowered and reduced degree enhanced with increasing relative humidity. To these brittle Eudragit® L100-55 in the dry state, enhancement of moisture can improve compressibility of pellets coated with Eudragit® L100-55 and avoid occurrence of crack.
2.2.3. Coating film thickness
Coating weight affects film thickness directly, and subsequently influences drug release. Coated pellets with thick films have strong toughness, but drug release from pellets probably be delayed. Under the circumstances, pore-foaming agent should be considered to be added (Table 2). Several tests indicated that suitable coating weights can meet different requirements of drug release on the condition of no destruction on coating films. If isolation layers are employed, thickness of isolation layer is determined according to the protection ability of isolation layers and drug release behavior.
Table 2.
Pore-foaming agents.
| Hydrophilic liquid | Glycerinum |
| PEG 200 | |
| Electrolyte | NaCl |
| KCl | |
| Na2SO4 | |
| Saccharides | Lactose |
| Fructose | |
| Saccharose | |
| Mannose | |
| Surfactant | Polysorbate 80 |
| Lauryl sodium sulfate | |
| Polymer | PEG |
| PVA | |
| Hydrogels | HPMC |
| CMC | |
| Tragacanth | |
| Foaming agent | Carbonate |
| Bicarbonate | |
2.3. Tabletting excipient
The selection of optimal excipients is essential to tabletting of pellets. There are several roles in the tabletting process: (i) It can be used to fill the space between pellets and reduce pressure from compression. (ii) It can prevent direct contact between pellets and mutual integration of polymeric films, which contribute to maintain integrity of coating film. (iii) It can be utilized to improve compressibility. (iv) Excipients with suitable size can reduce occurrence of separated pellets and excipients, improve uniformity of drug and decrease the difference of tablets weight. Excipients are usually added to mixtures in the form in the form of powder or granule (Altaf et al., 1999). Habib et al. (2002) prepared placebo beads being the same size with pellets by extrusion–spheronization followed by freeze drying to avoid segregation occurrence leading to weight variation and content uniformity problems. Several factors decide whether fillers can be used as tabletting excipient, including type, dosage, and physical and mechanical properties, such as elasticity, plasticity, and porosity. Torrado and Augsburger (1994) investigated the possible protective effect of different excipients on the tabletting of granules coated with Eudragit RS. The results showed that the order of least damage to the coating was: polyethylene glycol 3350 < microcrystalline cellulose < crospovidone < lactose < dicalcium phosphate. After compared with each other, suitable excipient mixture was: microcrystalline cellulose 50%, crospovidone 25% and polyethylene glycol 3350 25%. Under this mixture ratio, damage of the coating membranes was at very low compressional pressure and minimum damage.
Excipient properties, such as size, bulk density, surface morphology, and internal interaction, usually will influence mobility, segregation occurrence, tablet hardness and drug release behavior. With increasing demands for manufacturing technologies, several multifunctional excipients have been developed (Table 3). Some of them are good candidate for tabletting excipients. Due to the excellent elasticity coefficient and tensile strength, MCC is frequently used as compression of pellets. Ceolus KG-801 possesses the good compressibility and special high binding function in tablets, which is suitable for the tablets with higher hardness, low friability and even low content. Zeeshan et al. (2009) prepared and compacted pellets containing pseudoephedrine hydrochloride using Celous KG-801 granules and inert pellets as tabletting excipient. Because tensile strength of inert pellets was inferior to coated pellets, they were easy to crush during compression and subsequently serve as cushioning agent that protected the coating.
Table 3.
Commercially available co-processed excipient.
| Type | Brand name | Ingredients | Processing |
|---|---|---|---|
| Starch-based | Advantose® FS | Fructose-starch | Spray-drying |
| StarCap® 1500 | Corn starch-pregelatinized starch | Spray-drying | |
| Cellulose based | Avicel® HFE | MCC-Mannitol | Spray-drying |
| Avicel® RC-591 | MCC-Na CMC | Milling, spray-drying | |
| Avicel® RC-581 | MCC-Na CMC | Milling, bulk drying | |
| Avicel® CL-611 | MCC-Na CMC | Milling, spray-drying | |
| Barcroft® CS90 | Calcium carbonate-Starch | Spray-drying | |
| ForMaxx® | Calcium carbonate-Sorbitol | Spray-drying | |
| ProSolv® SMCC50 | MCC-Colloidal silicon dioxide | Spray-drying | |
| Xylitab®200 | Xylitol-Na CMC | Granulation | |
| Lactose-based | Cellactose® | α-Lactose monohydrate-Powder cellulose | Spray-drying |
| Microcellac® | α-Lactose monohydrate-MCC | Spray drying | |
| StarLac®100 | α-Lactose monohydrate-Corn starch | Spray drying | |
| Sugar-based | Di-Pac® | Sucrose-Maltodextrins | Co-crystallization |
| Compressol®S | Mannitol-Sorbitol | Melt extrusion | |
| LudiFlash® | Mannitol-PVA latex solids | Granulation | |
3. Technological factors
In addition to composition factors, technology is of great importance to compression of pellets likewise. The compaction process of coated pellets can be summarized in four stages:
-
(i)
Repositioning of pellets resulting in volume reduction of the pellet bed.
-
(ii)
Local surface deformation of pellets resulting in volume reduction of the pellet bed further.
-
(iii)
Bulk structure deformation of pellets resulting in densification.
-
(iv)
Low inter- and intragranular porosity contributing to ceased volume reduction.
3.1. Coating parameters
Fluid-bed coating technology is usually used as coating of pellets adopting spray gun in the bottom. Bottom spray system (known as Wurster system, Fig. 2) and air inlet are located at the bottom of the fluidized interface, which contributes to the same direction of hydrojet and materials movement. Continuous and regular movement of pellets, short distance that droplets reach the surface of the particles, as well as suitable drying efficiency contribute to the compact and continuous coating films. Technological factors affecting coating quality include feeding capacity, supply air rate, air temperature, atomizing pressure, spray rate, etc.
Figure 2.
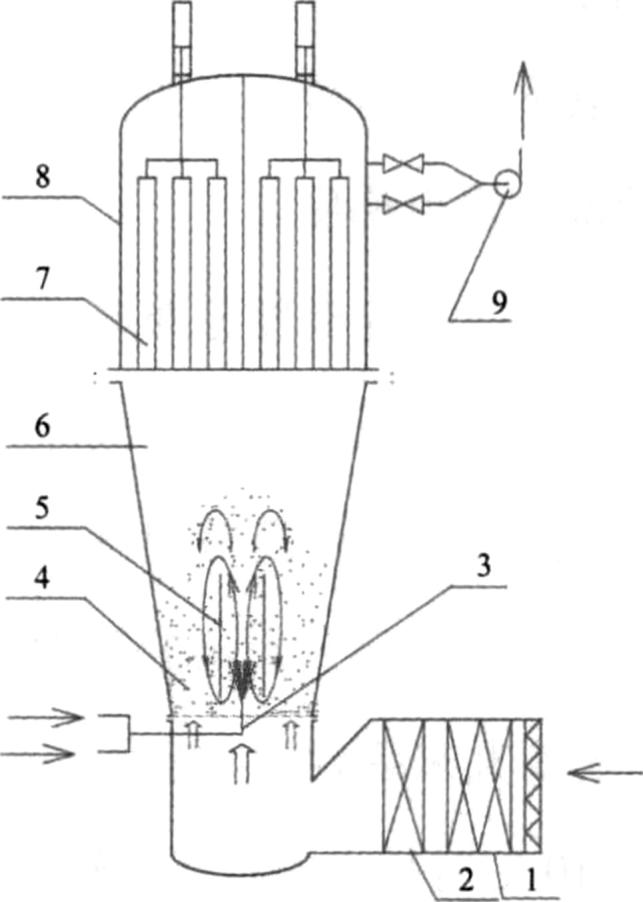
Schematic diagram of bottom spray fluidized bed. 1 – Inlet filter; 2 – heater; 3 – Spray gun in the bottom; 4 – Fluidized bed; 5 – Guide cylinder; 6 – Settling chamber; 7 – Strainer; 8 – Bartizan; 9 – Induced draft fan.
Feeding capacity usually decides fluidized state of pellets. Both too much and too little feeding capacity will reduce reproducibility and coating quality. The selection of feeding capacity must be based on the fluidized bed. Suitable fluidized stat can be kept by selecting supply air rate, which reduce material loss and enhance yield. Low supply air rate often result in the adhesion between pellets. On the contrary, too high supply air rate will increase friction and collision between pellets, which damages the coating films further. In addition, if air temperature exceeds Tg of coating materials, volatilization rate of solvent and coating efficiency will be improved substantially, but too high temperature will soften polymer extensively, cause adhesion between pellets, and even affect continuity of coating process.
Atomizing pressure and spray rate decide atomization effect of coating solution. Adhesion between pellets and Uneven coating are dependence with too low atomizing pressure or fast spray rate. Too high atomizing pressure not only cracks pellets, but also causes insufficient cover of coating solution. Spray rate is decided according to the different coating solution dispersion systems. Due to the low volatility and drying efficiency of aqueous dispersion, spray rate ought to be controlled at a slower speed and conducted to heal the coating films when necessary. Organic coating solution possesses fast solvent evaporation and high drying efficiency, but safety and residual solvents must be emphasized.
3.2. Tabletting parameters
Compression force usually affects hardness, friability and disintegration time of tablets. It is also related with drug release from coated pellets. Disintegration time and hardness of tablets will increase with the enhancement of compression force. If tabletting force is too small, friability of tablets will not be qualified. The selection of optimal compression force should be based on the properties of pellets and composition.
3.3. Others
Mixtures often delaminate in the industrial production process, which will not able to meet the requirements of content uniformity. In addition to consider relevant formulation factors, Feeding ways and quantities should also be investigated.
4. Conclusion
The challenges of formulating pellets into tablets are evident. Although many problems encountered in preparation technology restrict, to some extent, wide application of pellet-containing tablets, the dosage of tablets can be divided and persist original characteristics of drug release after segmentation, which provides flexible medication regimen for clinical usage. Both placid drug release in the human body and safe medication can be achieved, especially drugs with low therapeutic index, such as metoprolol tartrate, and carbamazepine.
So far, formulating pellets into tablets is still a challenge. Various materials and process-related parameters must be optimized to meet quality requirements. Thereinto, pellet cores, polymeric films and tabletting excipients must be investigated adequately. Hardness of pellets must be high enough to resist compression pressure. Meanwhile, good cushion elasticity can avoid fragmentation of pellets. The polymer coat must have the right combination of strength, ductility and thickness to withstand the forces generated during compaction without rupturing. Coating polymers that do not resist the mechanical stresses during compaction are not suitable for the preparation of coated pellets-containing tablets. The mechanical properties of the polymeric films and its response to the pressure must be studied deliberately. For tabletting excipient, its size and density should be similar to coated pellets as far as possible to reduce segregation. Secondly, the protection of excipient for the pellets should be considered. Determination of ratio between tabletting excipient and pellets must give consideration to the integrity of pellets and drug content in tablets. To achieve further development in compression technology of coated pellets, several novel methods are adopted to optimize preparation process, such as hot tabletting technique that stress from pellets tabletting under thermal condition is much less than general tabletting process and there are no consolidation effect and elastic recovery during compression in the meantime.
The development of pellet tabletting is closely related with the following aspects: ① The development of novel coating polymers. ② The development of precision tablet devices. ③ The development of novel tabletting excipient. With the fast development of material science and mechanical engineering, increasing research and application will occur in the pellet tabletting, and its industrialization can be achieved one day.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Xueying Tan, Email: tanxueying@163.com.
Jingbo Hu, Email: pandapig@zju.edu.cn.
References
- Abbaspour M.R., Sadeghi F., Afrasiabi Garekani H. Design and study of ibuprofen disintegrating sustained-release tablets comprising coated pellets. Eur. J. Pharm. Biopharm. 2008;68(3):747–759. doi: 10.1016/j.ejpb.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Abdul S., Chandewar A.V., Jaiswal S.B. A flexible technology for modified-release drugs: multiple-unit pellet system (MUPS) J. Control Rel. 2010;147(1):2–16. doi: 10.1016/j.jconrel.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Altaf S.A., Hoag S.W., Ayres J.W. Bead compacts. I. Effect of compression on maintenance of polymer coat integrity in multilayered bead formulations. Drug Dev. Ind. Pharm. 1998;24(8):737–746. doi: 10.3109/03639049809082721. [DOI] [PubMed] [Google Scholar]
- Altaf S.A., Hoag S.W., Ayres J.W. Bead compacts. II. Evaluation of rapidly disintegrating nonsegregating compressed bead formulations. Drug Dev. Ind. Pharm. 1999;25(5):635–642. doi: 10.1081/ddc-100102219. [DOI] [PubMed] [Google Scholar]
- Bashaiwoldu A.B., Podczeck F., Newton J.M. A study on the effect of drying techniques on the mechanical properties of pellets and compacted pellets. Eur. J. Pharm. Sci. 2004;21(2–3):119–129. doi: 10.1016/j.ejps.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Bendas E.R., Christensen J.M., Ayres J.W. Development and in vitro evaluation of mesalamine delayed release pellets and tableted reservoir-type pellets. Drug Dev. Ind. Pharm. 2010;36(4):393–404. doi: 10.3109/03639040903213717. [DOI] [PubMed] [Google Scholar]
- Bodmeier R., Paeratakul O. Mechanical properties of drug and wet cellulosic and acrylic polymer films prepared from aqueous colloidal polymer dispersions. Pharm. Res. 1994;11:882–888. doi: 10.1023/a:1018942127524. [DOI] [PubMed] [Google Scholar]
- Bornhöft M., Thommes M., Kleinebudde P. Preliminary assessment of carrageenan as excipient for extrusion/spheronisation. Eur. J. Pharm. Biopharm. 2005;59:127–131. doi: 10.1016/j.ejpb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Dashevsky A., Kolter K., Bodmeier R. Compression of pellets coated with various aqueous polymer dispersions. Int. J. Pharm. 2004;279(1–2):19–26. doi: 10.1016/j.ijpharm.2004.03.019. [DOI] [PubMed] [Google Scholar]
- EI-Malah Y., Nazzal S. Novel use of Eudragit NE 30D/Eudragit L 30D-55 blends as functional coating materials in time-delayed drug release applications. Int. J. Pharm. 2008;357(1-2):219–227. doi: 10.1016/j.ijpharm.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Ensslin S., Moll K.P., Metz H. Modulating pH-independent release from coated pellets: effect of coating composition on solubilization processes and drug release. Eur. J. Pharm. Biopharm. 2009;72:111–118. doi: 10.1016/j.ejpb.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Ghanam D., Kleinebudde P. Suitability of κ-carrageenan pellets for the formulation of multi particulate tablets with modified release. Int. J. Pharm. 2011;409(1–2):9–18. doi: 10.1016/j.ijpharm.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Goole J., Deleuze P., Vanderbist F. New levodopa sustained-release floating minitablets coated with insoluble acrylic polymer. Eur. J. Pharm. Biopharm. 2008;68:310–318. doi: 10.1016/j.ejpb.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Habib Y.S., Augsburger L.L., Shangraw R.F. Production of inert cushioning beads: effect of excipients on the physicomechanical properties of freeze-dried beads containing microcrystalline cellulose produced by extrusion-spheronization. Int. J. Pharm. 2002;233(1–2):67–83. doi: 10.1016/s0378-5173(01)00924-3. [DOI] [PubMed] [Google Scholar]
- Hosseini A., Körber M., Bodmeier R. Direct compression of cushion-layered ethyl cellulose-coated extended release pellets into rapidly disintegrating tablets without changes in the release profile. Int. J. Pharm. 2013;457(2):503–509. doi: 10.1016/j.ijpharm.2013.07.042. [DOI] [PubMed] [Google Scholar]
- Johansson B., Alderborn G. Degree of pellet deformation during compaction and its relationship to the tensile strength of tablets formed of microcrystalline cellulose pellets. Int. J. Pharm. 1996;132(1–2):207–220. [Google Scholar]
- Johansson B., Nicklasson F., Alderborn G. Effect of pellet size on degree of deformation and densification during compression and on compactability of microcrystalline cellulose pellets. Int. J. Pharm. 1998;163(1–2):35–48. [Google Scholar]
- Johansson B., Wikberg M., Alderborn G. Compression behavior and compactability of microcrystalline cellulose pellets in relationship to their pore structure and mechanical properties. Int. J. Pharm. 1995;117(1):57–73. [Google Scholar]
- Li J.G., Ye X.L., Yu K.L. Preparation and drug release in vitro of sustained-release pellet tablets of aspirin. Chin Hosp. Pharm J. 2012;32(18):1445–1449. [Google Scholar]
- Liu Y., Sun Y.H., Sun J. Preparation and in vitro/in vivo evaluation of sustained-release venlafaxine hydrochloride pellets. Int. J. Pharm. 2012;426:21–28. doi: 10.1016/j.ijpharm.2011.12.053. [DOI] [PubMed] [Google Scholar]
- Miller R.A., Leung E.M.K., Oates R.J. The compression of spheres coated with an aqueous ethylcellulose dispersion. Drug Dev. Ind. Pharm. 1999;25:503–511. doi: 10.1081/ddc-100102200. [DOI] [PubMed] [Google Scholar]
- Nicklasson F., Alderborn G. Modulation of the tabletting behaviour of microcrystalline cellulose pellets by the incorporation of polyethylene glycol [J] Eur. J. Pharm. Sci. 1999;9(1):57–65. doi: 10.1016/s0928-0987(99)00042-1. [DOI] [PubMed] [Google Scholar]
- Rujivipat S., Bodmeier R. Moisture plasticization for enteric Eudragit® L30D-55-coated pellets prior to compression into tablets [J] Eur. J. Pharm. Biopharm. 2012;81(1):223–229. doi: 10.1016/j.ejpb.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Sawicki W., Lunio R. Compressibility of floating pellets with verapamil hydrochloride coated with dispersion Kollicoat SR 30 D [J] Eur. J. Pharm. Biopharm. 2005;60(1):153–158. doi: 10.1016/j.ejpb.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Thommes M., Kleinebudde P. Use of kappa-carrageenan as alternative pelletisation aid to microcrystalline cellulose in extrusion/spheronisation. I. Influence of type and fraction of filler. Eur. J. Pharm. Biopharm. 2006;63:59–67. doi: 10.1016/j.ejpb.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Thommes M., Kleinebudde P. Use of kappa-carrageenan as alternative pelletisation aid to microcrystalline cellulose in extrusion/spheronisation. II. Influence of drug and filler type. Eur. J. Pharm. Biopharm. 2006;63:68–75. doi: 10.1016/j.ejpb.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Torrado J.J., Augsburger L.L. Effect of different excipients on the tableting of coated particles [J] Int. J. Pharm. 1994;106(2):149–155. [Google Scholar]
- Tunón A., Gråsjö J., Alderborn G. Effect of intragranular porosity on compression behaviour of and drug release from reservoir pellets. Eur. J. Pharm. Sci. 2003;19(5):333–344. doi: 10.1016/s0928-0987(03)00106-4. [DOI] [PubMed] [Google Scholar]
- Ye Z.W., Rombout P., Remon J.P. Correlation between the permeability of metoprolol tartrate through plasticized isolated ethylcellulose/hydroxypropyl methylcellulose films and drug release from reservoir. Eur. J. Pharm. Biopharm. 2007;67:485–490. doi: 10.1016/j.ejpb.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Zeeshan F., Peh K.K., Tan Y.T. Exploring the potential of a highly compressible microcrystalline cellulose as novel tabletting excipient in the compaction of extended-release coated pellets containing an extremely water-soluble model [J] AAPS Pharm. Sci. Tech. 2009;10(3):850–857. doi: 10.1208/s12249-009-9278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]