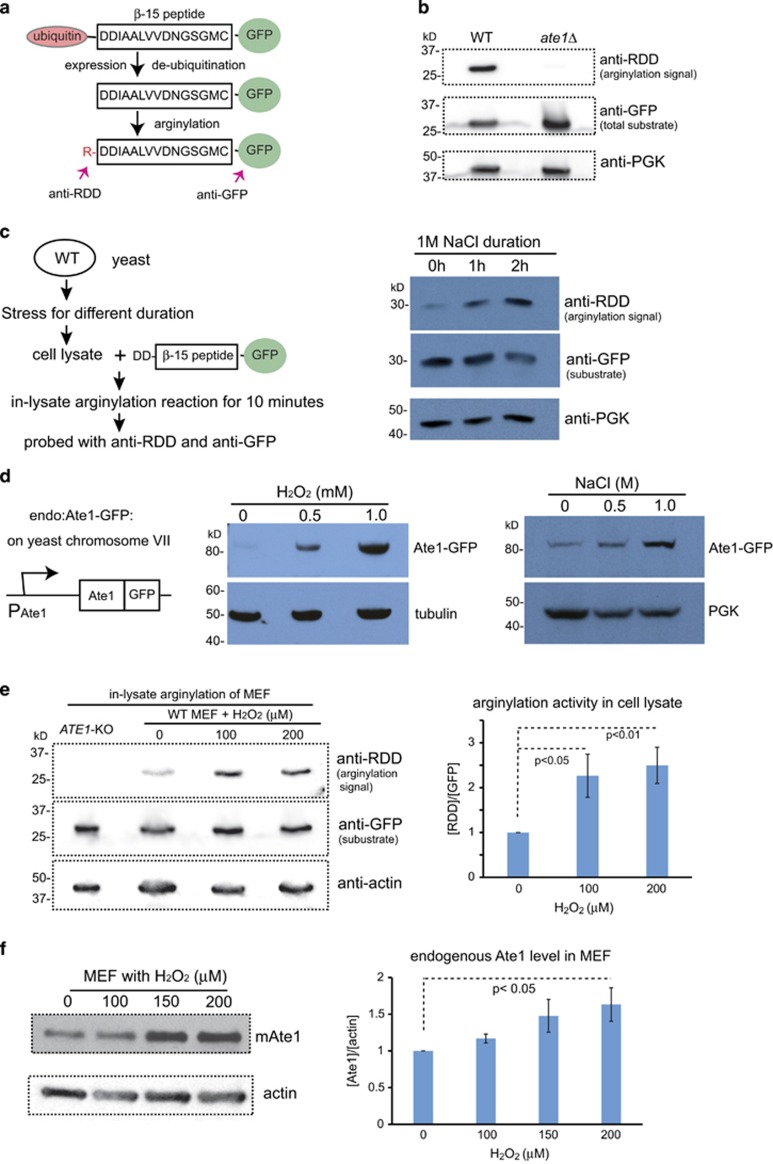
Figure 4.
The levels of Ate1 protein and global arginylation activity are upregulated during stress. (a) A scheme illustrating how DD-β15-GFP is used as the reporter of arginylation activity. The fusion protein containing a stretch of 15 amino acids starting with two aspartic acids (D) derived from the N terminus of mammalian β-actin, a known substrate of arginylation.31 This peptide is fused with an N-terminal ubiquitin, which is cleaved co-translationally by endogenous de-ubiquitylation enzymes in eukaryotic system and leaves the aspartic acids as the new N terminus. The arginylation state of this reporter can be probed with an anti-RDD antibody, which only reacts with the arginylated form of the reporter protein. A C-terminal GFP tag is used to facilitate the detection of steady-state level of the reporter protein by immunoblotting with anti-GFP antibody. (b) The arginylation level of DD-β15-GFP expressed in either WT or ate1Δ yeast was examined with anti-RDD antibody, which only shows a visible signal in the WT cells. An antibody for GFP was used to probe the total protein level of the DD-β15-GFP. PGK was used as a loading control for total yeast cellular proteins. (c) Illustrative scheme (left panel) and representative immunoblots (right panel) showing the arginylation activity in cell lysates prepared from yeast exposed to 1 M NaCl stress for increasing times. The lysates were then mixed with the recombinant protein DD-β15-GFP prepared from ate1Δ yeast for an in-lysate arginylation reaction for 10 min at RT. The arginylation level of the reporter protein was detected by immunoblotting with anti-RDD antibody. The steady-state level of the reporter protein was probed with anti-GFP. An anti-3-phosphoglycerate kinase (PGK) antibody was also used as a loading control. (d) On the left, a scheme illustrating the domain structure of the ‘in locus' GFP-fused Ate1, which is driven be the endogenous ATE1 promoter at the native chromosome locus (Chromosome VII) in the yeast (termed ‘endo: Ate1-GFP'). The right panels present immunoblots showing the steady-state levels of ‘endo: Ate1-GFP' in yeast treated with increasing concentrations of different stressors: H2O2 (left) or NaCl (right). Tubulin or PGK was used as loading controls. (e) WT MEFs were exposed to increased concentrations of H2O2 for 30 h. The lysates from all these cells, as well as untreated ATE1-KO MEFs (as a control), were then mixed with the recombinant protein DD-β15-GFP purified from ate1Δ yeast for an in-lysate arginylation reaction for 45 min at RT. The arginylation level of the reporter protein was detected by immunoblotting with anti-RDD antibody. The steady-state level of the reporter protein was probed with anti-GFP. Actin antibody was used as a loading control. The graph on the right side shows quantification from four independent repeats. (f) Left: representative immunoblots showing the levels of endogenous Ate1 proteins in MEFs treated with increasing concentrations of H2O2 for 30 h, detected by a specific antibody for mouse Ate1 (Millipore, clone 6F11) recognizing all four major Ate1-splicing variants. Actin was used as loading controls. Right: quantification of the endogenous Ate1 level in MEFs treated with H2O2 from three independent repeats. The Ate1 level was calculated by normalization with actin loading control, and then further normalized to the level at non-stressing condition (0 μM H2O2). In all above figures, error bars represent S.E.M. and statistical significance was calculated by Student's t-test