Figure 6.
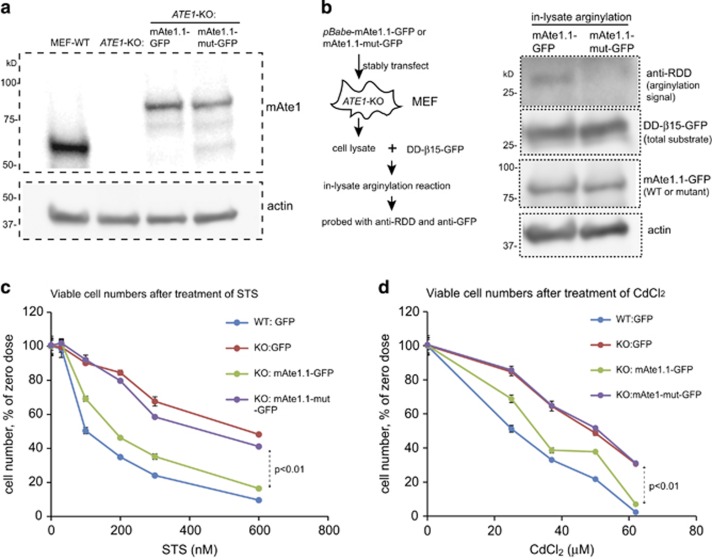
Mammalian Ate1 is required for cellular sensitivity to stressors in a manner dependent on its arginylation activity. (a) Representative immunoblots showing the levels of Ate1 protein detected by a specific mouse Ate1 antibody (Millipore, clone 6F11) as either endogenous Ate1 in WT MEF or the recombinant mouse Ate1-isofrom 1 (WT or C23-26S mutant) fused with a C-terminal GFP stably transfected in ATE1-KO cells. Actin was used as a loading control. (b) Left panel showing the procedure of using an in-lysate arginylation assay to measure the activities of either the WT version of mAte1.1-GFP or the mutant with cysteine 23 and 26 to serine replacement (referred as mAte1.1-mut-GFP in this study) expressed in stably transformed ATE1-KO MEF. The arginylation reporter protein, DD-β15-GFP, as described in Figure 4, was expressed and purified from ate1Δ yeast. On the right panel, anti-RDD was used to probe the level of arginylation on the reporter protein. Anti-GFP was used to show the total amount of reporter protein (DD-β15-GFP) added in each sample. Mammalian Ate1 antibody (Millipore, clone# 6F11) was used to detect the level of mAte1.1-GFP (either WT or mutant) present in each sample. Actin was used as a loading control for cell lysates. (c) Graph showing the quantification of cell viability after treatment of different concentrations of STS for 12 h, for WT and ATE1-KO cells stably expressing either GFP (as transfection and expression control), GFP-fused recombinant mouse Ate1.1 or enzymatically impaired mutant mouse Ate1.1. The viable cells were counted by cell counter and using Trypan blue to exclude dead cells. For each group, the data were normalized to a sample under non-stressing condition. Error bars represent S.E.M. (n=3). (d) Similar to (c), except that a treatment of CdCl2 was used. Error bars represent S.E.M. (n=3)