Fig. 9. Predicted pathway for P2Y2R/LRP1 interaction in the regulation of agLDL uptake.
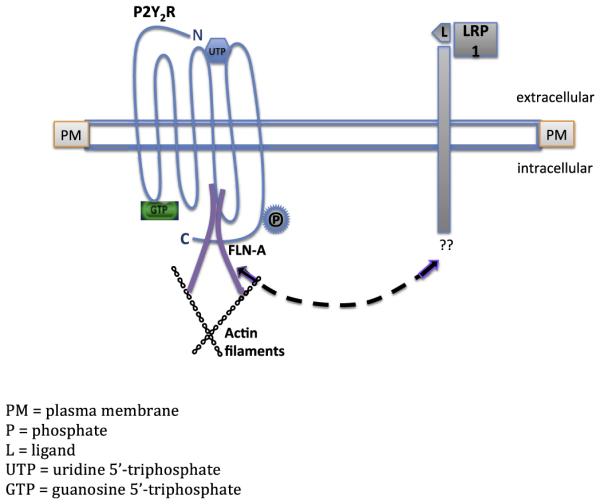
Diagram represents a proposed mechanism for P2Y2R-mediated uptake of agLDL in VSMCs and possible cross-talk between LRP1 and the P2Y2R. The LRP1 extracellular binding domain indicates binding of apo-E ligand (L). In vitro studies show that Apo E binding inhibits platelet-derived growth factor-induced migration and proliferation in smooth muscle cells (Boucher et al., 2003). We predict the existence of a regulatory element within the LRP1 promoter that is responsive to P2Y2R activation. We propose that LRP1 associates with the P2Y2R (dashed line) possibly via a phosphorylated residue in the C-terminus of the P2Y2R or through binding of LRP1 to P2Y2R-associated FLN-A and/or other cytoskeletal proteins. GTP indicates the activated state of the G protein-coupled P2Y2R.
