Figure 1.
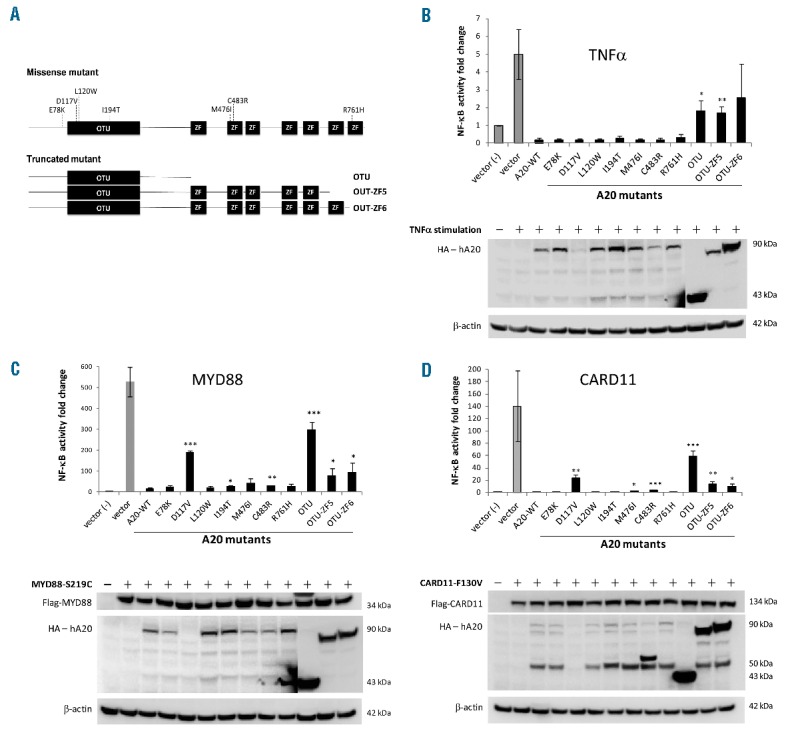
Functional characterisation of TNFAIP3 (A20) mutations. (A) The representative A20 mutants investigated by in vitro reporter assays, which include three truncation and 7 missense mutations that affect conserved amino acid residues. (B–D) NF-κB reporter assay shows that the wild type A20 is a potent inhibitor of NF-κB activation by TNFα, MYD88 and CARD11 mutants in HEK293T cells. All the three A20 truncation mutants consistently show a substantial impairment in suppression of NF-κB activation triggered by each of the three signalling pathways investigated, while the A20 missense mutants largely retain their ability to act as a global negative regulator of NF-κB despite some of the missense mutants displaying inconsistent evidence of impairment. The data is from three independent experiments and presented as a mean ± standard deviation, and the difference between A20 and its mutants is analysed by the unpaired student’s t-test. *P<0.05, **P<0.01, ***P<0.001. There is a significant difference in the level of protein expression between the A20 truncation and missense mutants in the reporter assays under stimulation by the MYD88 mutant and TNFα, and it is not possible to clearly illustrate their expression using the image from the same exposure time. Thus, the image for the A20 truncation mutants was taken from a shorter exposure (5 seconds), while the image for wild type A20 and its missense mutants was from a longer exposure (30 seconds).
