Abstract
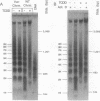
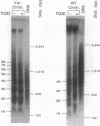
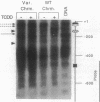
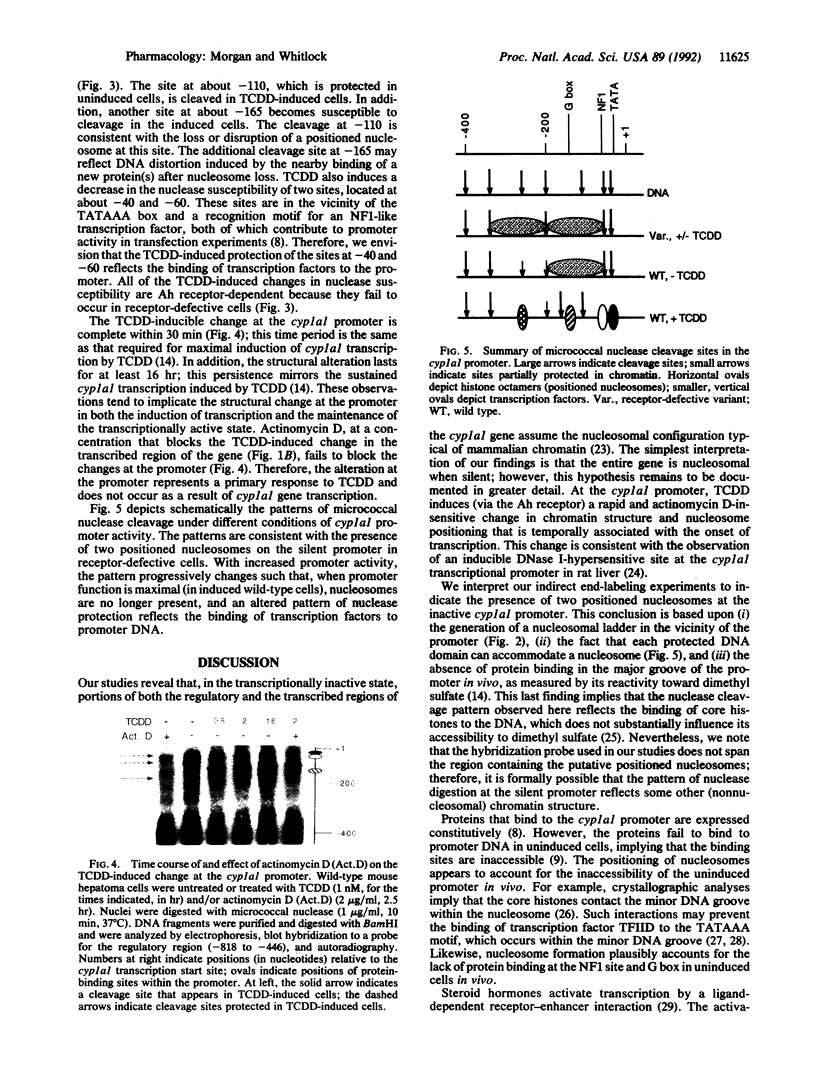
In mouse hepatoma cells, both the regulatory and the transcribed regions of the cyp1a1 gene assume a nucleosomal configuration when the gene is silent; two nucleosomes occupy specific sites at the transcriptional promoter. Activation of transcription by 2,3,7,8-tetrachlorodibenzo-p-dioxin is accompanied by changes in chromatin structure, which depend upon a functional aromatic hydrocarbon (Ah) receptor. In the transcribed region of the gene, nucleosome disruption occurs as a consequence of RNA elongation. In contrast, at the promoter, loss of positioned nucleosome sis independent of transcription and represents an event in the mechanism by which the liganded Ah receptor enhances transcriptional initiation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailar J. C., 3rd How dangerous is dioxin? N Engl J Med. 1991 Jan 24;324(4):260–262. doi: 10.1056/NEJM199101243240409. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Bellard M., Dretzen G., Bellard F., Oudet P., Chambon P. Disruption of the typical chromatin structure in a 2500 base-pair region at the 5' end of the actively transcribed ovalbumin gene. EMBO J. 1982;1(2):223–230. doi: 10.1002/j.1460-2075.1982.tb01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordingley M. G., Riegel A. T., Hager G. L. Steroid-dependent interaction of transcription factors with the inducible promoter of mouse mammary tumor virus in vivo. Cell. 1987 Jan 30;48(2):261–270. doi: 10.1016/0092-8674(87)90429-6. [DOI] [PubMed] [Google Scholar]
- Dong F., van Holde K. E. Nucleosome positioning is determined by the (H3-H4)2 tetramer. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10596–10600. doi: 10.1073/pnas.88.23.10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrin L. K., Weber J. L., Gorski J. Chromatin structure, transcription, and methylation of the prolactin gene domain in pituitary tumors of Fischer 344 rats. J Biol Chem. 1984 Jun 10;259(11):7086–7093. [PubMed] [Google Scholar]
- Durrin L. K., Whitlock J. P., Jr 2,3,7,8-Tetrachlorodibenzo-p-dioxin-inducible aryl hydrocarbon receptor-mediated change in CYP1A1 chromatin structure occurs independently of transcription. Mol Cell Biol. 1989 Dec;9(12):5733–5737. doi: 10.1128/mcb.9.12.5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elferink C. J., Whitlock J. P., Jr 2,3,7,8-Tetrachlorodibenzo-p-dioxin-inducible, Ah receptor-mediated bending of enhancer DNA. J Biol Chem. 1990 Apr 5;265(10):5718–5721. [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin as an essential part of the transcriptional mechanism. Nature. 1992 Jan 16;355(6357):219–224. doi: 10.1038/355219a0. [DOI] [PubMed] [Google Scholar]
- Grunstein M. Histone function in transcription. Annu Rev Cell Biol. 1990;6:643–678. doi: 10.1146/annurev.cb.06.110190.003235. [DOI] [PubMed] [Google Scholar]
- Hapgood J., Cuthill S., Söderkvist P., Wilhelmsson A., Pongratz I., Tukey R. H., Johnson E. F., Gustafsson J. A., Poellinger L. Liver cells contain constitutive DNase I-hypersensitive sites at the xenobiotic response elements 1 and 2 (XRE1 and -2) of the rat cytochrome P-450IA1 gene and a constitutive, nuclear XRE-binding factor that is distinct from the dioxin receptor. Mol Cell Biol. 1991 Sep;11(9):4314–4323. doi: 10.1128/mcb.11.9.4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel D. I., Whitlock J. P., Jr Regulation of cytochrome P1-450 gene transcription by 2,3,7, 8-tetrachlorodibenzo-p-dioxin in wild type and variant mouse hepatoma cells. J Biol Chem. 1984 May 10;259(9):5400–5402. [PubMed] [Google Scholar]
- Jones K. W., Whitlock J. P., Jr Functional analysis of the transcriptional promoter for the CYP1A1 gene. Mol Cell Biol. 1990 Oct;10(10):5098–5105. doi: 10.1128/mcb.10.10.5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg R. D., Lorch Y. Irresistible force meets immovable object: transcription and the nucleosome. Cell. 1991 Nov 29;67(5):833–836. doi: 10.1016/0092-8674(91)90354-2. [DOI] [PubMed] [Google Scholar]
- Laybourn P. J., Kadonaga J. T. Role of nucleosomal cores and histone H1 in regulation of transcription by RNA polymerase II. Science. 1991 Oct 11;254(5029):238–245. doi: 10.1126/science.254.5029.238. [DOI] [PubMed] [Google Scholar]
- Lee D. K., Horikoshi M., Roeder R. G. Interaction of TFIID in the minor groove of the TATA element. Cell. 1991 Dec 20;67(6):1241–1250. doi: 10.1016/0092-8674(91)90300-n. [DOI] [PubMed] [Google Scholar]
- Lohr D. The chromatin structure of an actively expressed, single copy yeast gene. Nucleic Acids Res. 1983 Oct 11;11(19):6755–6773. doi: 10.1093/nar/11.19.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. D., Felsenfeld G. Reaction of nucleosome DNA with dimethyl sulfate. Proc Natl Acad Sci U S A. 1979 May;76(5):2133–2137. doi: 10.1073/pnas.76.5.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Israel D., Whitlock J. P., Jr Biochemical and genetic analysis of variant mouse hepatoma cells defective in the induction of benzo(a)pyrene-metabolizing enzyme activity. J Biol Chem. 1983 Mar 25;258(6):3523–3527. [PubMed] [Google Scholar]
- Nedospasov S. A., Georgiev G. P. Non-random cleavage of SV40 DNA in the compact minichromosome and free in solution by micrococcal nuclease. Biochem Biophys Res Commun. 1980 Jan 29;92(2):532–539. doi: 10.1016/0006-291x(80)90366-6. [DOI] [PubMed] [Google Scholar]
- Negishi M., Swan D. C., Enquist L. W., Nebert D. W. Isolation and characterization of a cloned DNA sequence associated with the murine Ah locus and a 3-methylcholanthrene-induced form of cytochrome P-450. Proc Natl Acad Sci U S A. 1981 Feb;78(2):800–804. doi: 10.1073/pnas.78.2.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmann T., Wrange O. Specific glucocorticoid receptor binding to DNA reconstituted in a nucleosome. EMBO J. 1988 Oct;7(10):3073–3079. doi: 10.1002/j.1460-2075.1988.tb03172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piña B., Brüggemeier U., Beato M. Nucleosome positioning modulates accessibility of regulatory proteins to the mouse mammary tumor virus promoter. Cell. 1990 Mar 9;60(5):719–731. doi: 10.1016/0092-8674(90)90087-u. [DOI] [PubMed] [Google Scholar]
- Poland A., Glover E., Kende A. S. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem. 1976 Aug 25;251(16):4936–4946. [PubMed] [Google Scholar]
- Poland A., Knutson J. C. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Rannug A., Rannug U., Rosenkranz H. S., Winqvist L., Westerholm R., Agurell E., Grafström A. K. Certain photooxidized derivatives of tryptophan bind with very high affinity to the Ah receptor and are likely to be endogenous signal substances. J Biol Chem. 1987 Nov 15;262(32):15422–15427. [PubMed] [Google Scholar]
- Richmond T. J., Finch J. T., Rushton B., Rhodes D., Klug A. Structure of the nucleosome core particle at 7 A resolution. Nature. 1984 Oct 11;311(5986):532–537. doi: 10.1038/311532a0. [DOI] [PubMed] [Google Scholar]
- Safe S. H. Comparative toxicology and mechanism of action of polychlorinated dibenzo-p-dioxins and dibenzofurans. Annu Rev Pharmacol Toxicol. 1986;26:371–399. doi: 10.1146/annurev.pa.26.040186.002103. [DOI] [PubMed] [Google Scholar]
- Simpson R. T. Nucleosome positioning: occurrence, mechanisms, and functional consequences. Prog Nucleic Acid Res Mol Biol. 1991;40:143–184. doi: 10.1016/s0079-6603(08)60841-7. [DOI] [PubMed] [Google Scholar]
- Starr D. B., Hawley D. K. TFIID binds in the minor groove of the TATA box. Cell. 1991 Dec 20;67(6):1231–1240. doi: 10.1016/0092-8674(91)90299-e. [DOI] [PubMed] [Google Scholar]
- Turner B. M. Histone acetylation and control of gene expression. J Cell Sci. 1991 May;99(Pt 1):13–20. doi: 10.1242/jcs.99.1.13. [DOI] [PubMed] [Google Scholar]
- Whitlock J. P., Jr Genetic and molecular aspects of 2,3,7,8-tetrachlorodibenzo-p-dioxin action. Annu Rev Pharmacol Toxicol. 1990;30:251–277. doi: 10.1146/annurev.pa.30.040190.001343. [DOI] [PubMed] [Google Scholar]
- Wu C. The 5' ends of Drosophila heat shock genes in chromatin are hypersensitive to DNase I. Nature. 1980 Aug 28;286(5776):854–860. doi: 10.1038/286854a0. [DOI] [PubMed] [Google Scholar]
- Wu C., Wong Y. C., Elgin S. C. The chromatin structure of specific genes: II. Disruption of chromatin structure during gene activity. Cell. 1979 Apr;16(4):807–814. doi: 10.1016/0092-8674(79)90096-5. [DOI] [PubMed] [Google Scholar]
- Wu L., Whitlock J. P., Jr Mechanism of dioxin action: Ah receptor-mediated increase in promoter accessibility in vivo. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4811–4815. doi: 10.1073/pnas.89.11.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]