Abstract
Background An increasing array of rare inherited conditions can be detected as part of the universal newborn screening programme. The introduction and evaluation of these service developments require consideration of the ethical issues involved and appropriate mechanisms for informing parents and gaining consent if required. Exploration of parental views is needed to inform the debate and specifically consider whether more flexible protocols are needed to fit with the public perception of new developments in this context.
Objective This study has been undertaken to explore perceptions and attitudes of parents and future parents to an expanded newborn screening programme in the United Kingdom and the necessary information provision and consent processes.
Design and participants A mixed methods study involving focus groups (n = 29) and a web‐survey (n = 142) undertaken with parents and future parents.
Results and conclusions Parents want guaranteed information provision with clear decision‐making powers and an awareness of the choices available to them. The difference between existing screening provision and expanded screening was not considered to be significant enough by participants to warrant formal written, informed consent for expanded screening. It is argued that the ethical review processes need to be more flexible towards the provision of information and consent processes for service developments in newborn screening.
Keywords: expanded newborn screening, heel‐prick test, informed consent
Introduction
In the United Kingdom, newborn screening is routinely offered for a number of conditions through testing of a ‘heel‐prick’ blood spot taken in the first week after birth. 1 As a result of technological development and specifically the potential of tandem mass spectrometry, 2 rapid detection of a much larger number of inherited metabolic diseases is possible early in a child’s life. This approach has now become widely adopted in many developed countries, and the effectiveness of these programmes is currently under evaluation. All states in the USA now screen for 53 core conditions as part of their Federal programme, and many countries within the EU screen for between 2 and 19 such disorders.
In the United Kingdom, before screening for a condition is adopted by the national screening programme, the proposal is properly subject to a comprehensive assessment of the likely benefits, harms and costs of screening. 1 , 3 Currently, a proposal is being considered to include five additional inherited disorders, with a collective incidence of approximately 1:30 000 live births; they include the following: glutaric aciduria type 1, 4 isovaleric acidaemia, 5 non‐pyridoxine responsive homocystinuria, 6 maple syrup urine disease 7 and long‐chain hydroxyl acyl CoA dehydrogenase deficiency. 8 Whilst of enormous benefit to the children detected, such developments undertaken to evaluate the expansion of screening delivered, require consideration of not only the clinical and financial issues involved, but also the ethical issues for the whole population.
Screening, whether as part of service provision or research, requires participant consent processes. 9 , 10 For current newborn screening provision in the United Kingdom, this is through a process of informed choice or dissent, whereby a parent is expected to make a rational and informed choice regarding their child’s health, and is entitled to opt‐out of the national screening programme. 9 It is important for any service developments that similar safeguards are in place and that parents receive an appropriate level of information to enable an informed choice on behalf of their child. Here, the model of consent for an expanded screening programme in the United Kingdom is considered.
UK research suggests that voluntary informed choice may not always be obtained for newborn screening under service provision. 11 Screening is often experienced as a routine test, where parents are not even aware that they have a clear choice to make. 12 An expanded programme, where the range of screened conditions is extended to very rare conditions, raises questions regarding the amount and type of information parents need to consider consenting to their child’s inclusion. Gaining consent is challenging where there may be conflict between parental choice, efficient service provision and health promotion. 13 Fully informed written consent for expanded screening presents challenges, 14 , 15 which may impede development, burden health professionals and could reduce participation in the core screening programme. 11 , 14 It may limit sample sizes and create barriers to techniques that are safe and provide health benefits, whilst adding cost and logistical complexity. 13 Given the rarity and complexity of the conditions for which screening is possible, as well as the complexity of the screening process, educating parents to meet the criteria for fully informed consent is likely to be very difficult. 16 , 17 , 18
There is an assumption that by providing the right information to parents, they will make an ‘informed’ choice. However, this ignores individual differences in decision making and the impact of social and cultural factors that influence whether information can be translated into effective decision making at the individual level. 19 Decision‐making ability can be compromised by anxiety, dependence on and trust in the medical system and the challenge of new parenthood, as well as parent’s ability to read and retain information. 20 Further issues of information overload, parental anxiety, existing ‘psychological commitment’ to the test and providing insufficient information, are all valid concerns. 16 , 21
Whilst parental understanding and reassurance is paramount, Helgesson et al. 22 argue that legal regulations and ethical guidelines often suggest quite advanced understanding of information is necessary for informed consent. They counter that a basic understanding should ethically be sufficient: that it is up to the parent to decide, that they have an unrestricted right to withdraw consent at any time and realize the expected benefits and costs (in terms of risks and time). 23 , 24
Documenting informed consent through consent forms and signatures does not ensure informed decision making. 22 Tarini et al. 25 argue the focus should be on effective communication and education about screening rather than gaining written individual consent, although the opportunity to opt‐out should always be available. Whilst ethicists suggest that opt‐out strategies can reduce participant autonomy, opt‐in recruitment strategies have been shown to result in lower response rates and a biased sample. 26 Studies exploring patient and maternal satisfaction have shown equal satisfaction whether an opt‐in or opt‐out procedure is employed. 27 Written consent has been shown to be less important to the participants when the development was considered a continuation of an already initiated clinical service (such as screening). 14 However, it is noteworthy that in Germany, an expanded newborn screening programme employing written parental consent still demonstrated high compliance. 28
Within the context of UK expanded newborn screening, it is argued that exploration of parental views on consent practices is needed to further inform the debate and consider whether more flexible procedures are needed to fit with the public perception of the expansion and the opportunity to develop the service. To date, this has received little consideration. 13 Parental views and preferences have been analysed in respect to routine screening provision. 29 , 30 , 31 , 32 These studies have demonstrated that parents have limited knowledge about newborn screening practices and often are not aware of having provided explicit consent. The need for clear, brief and accurate patient information that takes into account parents needs has been highlighted. 12 , 13 Detmar et al. specifically considered views on the expansion of the neonatal screening programme in the Netherlands. 31 , 32 The medical benefits of screening were recognized, and almost 100% of their sample would participate. The parents held mixed views on whether informed consent was necessary. Here, a study of the views of UK parents and parents‐to‐be are considered in respect to the expansion of newborn screening, information provision and consent practices.
Methods
A combined methodology was employed involving focus groups and an online survey run in parallel. Ethical approval was sought and granted by the Coventry University Ethics Committee.
Online survey
The anonymous web‐based survey enabled efficient collection and analysis of data from a larger, dispersed sample. It offered the advantages of minimizing social desirability and interview bias, whilst allowing quantification of results.
Sampling
The survey was widely and publicly accessible on the internet, although it was expected that the majority of respondents would be parents or expectant parents. A free prize draw of £100 of shopping vouchers was used to encourage participation. The survey link was distributed through various mailing lists (e.g. the local council, local Children’s Centres) as well as postings on parental support groups. Distribution was particularly aimed at gaining the views of parents of children affected by inherited conditions, and a number of organizations (e.g. Cystic Fibrosis Trust, CLIMB and Genetic Interest Group) circulated the survey link and advertised the project on their websites. A relatively small motivated sample was expected owing to the subject matter and length of the survey.
The survey was fully completed by 142 participants (see Table 1) for gender and parental status summary). A further 45 participants submitted incomplete surveys; these were removed from the analysis.
Table 1.
Characteristics of survey participants
| Survey sample | Focus group sample | |
|---|---|---|
| Gender | ||
| Male | 16 | 2 |
| Female | 124 | 27 |
| Parental status | ||
| Parents of healthy child | 108 | 24 |
| Parents‐to‐be | 6 | 5 |
| Parents of child with disorder | 13 | 0 |
| None of the above | 15 | 0 |
Data collection and analysis
The survey was run using SurveyMonkey.com software. 33 Following a brief demographics section (10 questions), the survey consisted of 36 questions for all participants. The majority of the questions were multiple choice, with nine open questions asking for more detailed responses. Completion took approximately 30 min.
The questions addressed participants’ experiences of screening, the information given and the mechanism by which consent was obtained. Expanded newborn screening was explained as well as the difference between routine screening as part of existing service provision and the expanded newborn screening programme. A potential participant information sheet for expanded newborn screening was embedded within the survey. The leaflet, developed with metabolic condition specialists, was an adapted version of the ‘Newborn blood spot screening for your baby’ leaflet produced by the UK Newborn Screening Programme Centre. 34 The main addition to the leaflet was a section specifically referring to inherited metabolic diseases which could potentially be the focus of an expanded screening programme. The participants were asked to read the information as if they were deciding whether they would like their child to take part and then asked a series of questions. Questions were also posed about willingness to take part in the programme and appropriate means of providing consent.
As this was an exploratory survey investigating experience, attitudes and perceptions, descriptive statistics rather than tests of statistical inference were undertaken.
Focus groups
The focus groups allowed more in‐depth discussion of issues with harder to reach populations, who may have been less likely to access an online survey.
Participants
Participants were recruited through local children’s centres and antenatal groups. Four focus groups and three interviews were undertaken with 29 participants in total. This included individuals with children who had experienced newborn screening, as well as future parents. Participants were provided with refreshments and a £10 shopping voucher for attending the session.
The aim was to engage groups with potentially different viewpoints and experiences, although each focus group comprised of participants with similar socio‐economic backgrounds to encourage group dynamics. As illustrated in Table 2, this included new parents who had recently experienced newborn screening (within the previous 10 weeks); parents who had experienced screening within the last 2 years; and mothers of a South Asian origin (children all under 2 years). One of two groups run with South Asian mothers was conducted in Urdu and translated into English. Owing to difficulties experienced in getting expectant parents together for a focus group, three interviews were undertaken with expectant parents in the final trimester: two were run with couples and one on a one‐to‐one basis.
Table 2.
Focus group participants
| Number of participants | Ethnic origin | Age of children | Recruited through | |
|---|---|---|---|---|
| Focus group 1 | 10 | White British | Under 2 years | Local children’s centre |
| Focus group 2 | 5 | South Asian (fluent English speakers) | Under 2 years | Local children’s centre |
| Focus group 3 | 3 | South Asian (non‐English speakers) | Under 2 years | Local children’s centre |
| Focus group 4 | 6 | Mixed ethnic origin | Under 10 weeks | National Childbirth Trust |
| Interviews | 5 | White British | Expectant parents in the final trimester of pregnancy | National Childbirth Trust |
Data collection and analysis
Each focus group and interview lasted between 30 and 90 min. The discussions were directed using a standard schedule and material for discussion. Whilst the approach offered some structure, it was possible to discuss issues as they were raised by participants. Discussion topics included participants’ experiences of screening, the information given and the mechanisms by which consent was obtained. Expanded newborn screening was explained to the participants. The newborn screening leaflet as described above was presented for discussion. Participants were asked to read and review the information provided in terms of its content, readability and suitability. The concepts of informed dissent and consent were then explained and their appropriateness to the proposed programme discussed.
The discussions were recorded and transcribed. Transcripts were analysed as separate groups by a researcher and the data subjected to thematic analysis in accordance with Braun and Clarke. 35 An inductive approach was used to identify predominant themes across the data and provide confidence that the emerging findings were not obscured by existing evidence and theory. Accordingly, the researcher was unfamiliar with literature on screening and informed consent at the time of data collection and analysis. Following line‐by‐line coding, themes were identified at the semantic level, that is, the analytical focus was on explicit surface meanings of the data and not on looking for anything beyond what the respondents said. Transcripts were coded to identify relevant aspects of the data, once a comprehensive set of codes had been identified; repeated patterns across the data set were identified to generate themes. These themes were then reviewed and refined and illustrative quotations selected.
Results
Experiences of routine screening
Twenty‐four of twenty nine focus group participants and 92.6% of surveyed parents (79% of total sample) had children who had been screened in the United Kingdom. Most of the parents had little recollection of specifically what the testing had been for. The experiences recalled by focus group parents included whether their partner had been present (which was often not the case) and whether the heel‐prick caused any distress. A feeling of distress as the blood was taken was not uncommon:
yeah, I cried my eyes out
(P7 L11)
Twenty‐one percent of parents completing the survey agreed that they were anxious about the safety of their child during the heel‐prick. Midwife advice appeared to determine how prepared the mother was.
Discontentment amongst focus group participants concerned the provision of screening results. Experiences were inconsistent, with some receiving results by letter, often later than expected, and some assuming a negative result having not had any contact. In two of the focus groups, all participants reported no notification of results:
..they said they would send the results to the doctor but when I asked the doctor they said no we haven’t got that, and we have not got it written in our book as well
(P5, A96‐97)
All parents agreed that they would have liked prompt and formal receipt of results for reassurance.
Of the survey sample, 61.2% remembered being given information about screening before the blood spot was taken; 19.8% claimed not to have received any. It is hard to determine whether information was not provided, or simply not recognized or read. Discussion within the focus groups indicated that the information varied from none, to a leaflet, to a combination of a conversation with the midwife and written information. Many participants admitted receiving a leaflet but not having read it:
I put the leaflets in my bag and never read them
(P7, L239)
The verbal information provision by the midwife was often minimal:
…with my experience I wasn’t given anything, she just came over and said we are going to do a heel‐prick test, and I said ok fine
(P1, A16‐17)
Minimal information was not necessarily considered a problem at the time, but on reflection parents indicated that more information or attention to the information provided would have been useful.
Whilst subject to variation, information about newborn screening should be provided at the 8‐week antenatal appointment, after birth and directly before the test. Focus group and survey participants (65%) mainly recalled information presentation to be after birth. This was considered inappropriate:
...after you have had a kid you have got so much on your mind that you don’t want to look through hundreds and tons of leaflets
(P3 A121‐22)
It was felt that first time provision of information should have been during pregnancy, with refreshers after birth and before the test (as is intended).
Of those survey participants who could remember the information, 65.8% thought the quantity was appropriate, and 62.2% indicated that they had understood it. However, participants felt they had poor knowledge and understanding of which conditions were being tested for and what they would mean for their child.
Of survey participants, 51.1% were aware that screening is optional. When reflecting on their own experiences, 41.7% reported that they did not feel able to decline from having the blood spot taken, with many believing screening was compulsory:
No – thought it was compulsory, didn’t realise I had a choice and therefore a decision to make
(SQ30, P72)
Focus group participants agreed; in many cases, screening was not presented as optional, and an assumption was made that their child would be screened:
..all the ones [screening tests] after birth, it is assumed that they are just going to be done there is no choice about it. ‘I am going to do the heel‐prick now’. It is the way it is worded
(P5 S97‐98)
This experience was common irrespective of the background of participants. However, most parents did not question the decision to have their child screened, with 80.7% of survey participants reporting that they had never experienced doubts that the correct decision was made.
Views on expanded newborn screening
The participants were described an expanded newborn screening programme where the same heel‐prick blood spot taken for routine screening would be being tested for a further five inherited metabolic conditions.
Information requirements
Participants were asked whether an expanded screening programme should provide different information to routine screening. Of survey participants, 44.6% wanted more information and 51.1% the same amount. An expansion of screening could involve complex and rare conditions. Participants were asked whether they would require the same quantity of information for rare conditions as they would for more common and familiar conditions. There was a difference in opinion demonstrated through both the survey and focus group. The same amount of information was required by 47.9%, more by 20% and less by 26.4%. Only 2.9% thought no information would be required. Some felt less information was appropriate to prevent undue concern, whilst others felt more was needed to explain unfamiliar conditions.
Review of expanded newborn screening leaflet
Following review of the adapted ‘Newborn blood spot screening for your baby’ leaflet, survey participants were asked to rate ease of understanding of the information presented, and how well the conditions were described. The responses were indicated on a five‐point Likert scale (strongly disagree through to strongly agree). The results are summarized in Fig. 1, and it can be seen that the information was positively regarded.
Figure 1.
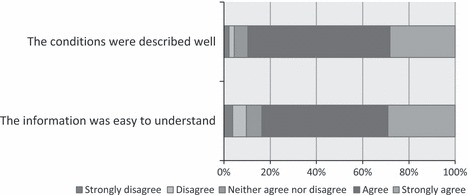
Review of the newborn screening leaflet.
Focus group participants held differing views of the leaflet. Some were positive, feeling that it contained the right level of information. Others would have liked to be told more about the process of the screening and the reasons for the expansion of the programme. The leaflet was criticized by two of the focus groups for being too complicated because of the terminology used:
You see all these words…it’s a bit complicated
(P7, L223)
…you can’t understand it just by reading the leaflet
(P1 U75‐6)
Some criticized the leaflet for causing anxiety, by stating that screened conditions could result in death. Many felt there was too much information presented:
I think that too much information is actually more harmful than giving much less information
(P3 S208)
Participants agreed that they would initially like to receive basic information on screening, with access to more detailed resources and the capacity to individually select the additional information that was meaningful to them. A more detailed and lengthy leaflet was not required, instead brief paper‐based reading material with the option to consult more detailed written or web‐based information, or contact a health professional.
Participant preference for face‐to‐face discussions with a health professional, typically the midwife was clear. This was particularly evident amongst the focus group participants from an Asian background, for whom English was not their first language.
Timing of information provision
When asked to consider when they would want to receive information about expanded newborn screening in order to make a decision about their child’s inclusion, the most common response was during late pregnancy. Other responses suggested during pregnancy, as well as after giving birth and before the tests. This is consistent with parents reflection’s on their experiences of routine screening. Presentation late in pregnancy would provide time and attention to be devoted to the information and further reading if required, before becoming overwhelmed by the arrival of a new baby:
Yeah, probably after thirty weeks but before 38 weeks
(P1 PL321)
Both the survey and focus group feedback suggested that a reminder was needed 5–7 days before and directly before the test. This would allow for increased saliency of information and changing views after the birth, and provide time to digest additional information if required.
Decision making
The survey participants were asked whether they could make a decision to include their child in the programme based on the presented leaflet, and 90.1% of respondents indicated that they could. Those unable to make a decision wanted more information, specifically about the programme, what was being tested for and the accuracy of the tests. Assuming the right amount of information, 42.3% of survey participants felt they would make the decision to include their child in an expanded screening programme straightaway; 57.7% felt they would need time to decide. The majority would need between a day and a week to reach a decision.
Choice and consent
Freedom of choice in expanded screening was desired by all focus group participants:
I would like to know all the facts and make an informed choice about what I want to do going forward
(P5 L411‐12)
The risk that some parents may not act in the best interests of their child was raised, but choice was still important. Most parents did not consider the option of including their child in routine screening, yet declining from the screening of additional conditions. Expanded screening was seen as interlinked with routine screening, with a broad acceptance of the proposed programme.
Potential formats for providing and recording consent were considered. A system of opting out of the screening programme was seen to be desirable by 71.2% of survey participants, 27.3% preferring opt‐in and 1.4% not recognizing the difference. Focus group discussion suggested that a formal process involving opting‐in and written consent, if not required for routine screening, would arouse suspicion and mistrust, causing extra worry and making the decision harder.
I wouldn’t want to sign something because it makes it seem more formal and you have to think more about it, ……. but you always know that you have got the chance to object
(P10, L208‐310)
Fully informed written consent procedures were almost unanimously considered unnecessary. Choice was considered essential, but signing for it was not.
Willingness to take part
All of the focus group participants and 92.3% of survey participants indicated that based on the information provided, they would be prepared to include their child in an expanded screening programme, and 7.7% would not be happy to take part. The programme was perceived as low risk as additional blood was not being taken, and nothing was being introduced to the child’s body. Therefore, most parents and future parents could only see the benefits, especially of gaining any additional knowledge about their child’s health.
Differences amongst groups
No clear differences were found in opinions about the information provision or consent methods for expanded screening between parents and future parents, or between focus groups based on participant background that is by area or ethnic origin. The survey sample included a small number of parents with children affected by conditions for which screening is currently undertaken or possible (n = 13). In 10 of 13 of these cases, diagnosis occurred as a result of newborn screening. There were no clear differences in the views of these parents. However, their preferences for clear information provision during pregnancy and their commitment to expanded screening were evident.
Discussion
This research has examined the views of parents and future parents, on information provision and consent processes for expanded newborn screening.
Experiences of routine screening
Consideration of parental experiences sought to identify, where the current screening process may need review if an expanded programme of screening were to be run in parallel. In general, the heel‐prick screening test was seen as routine; despite limited knowledge, parents were confident in the potential health benefits. This is consistent with existing research in the area. 12 , 31 It is clear that communication of expectations and consistent follow‐up in terms of results is required. In expanded screening provision, preparation for any perceived negatives of the testing process will be important to ensure parental satisfaction with their experience.
Information provision
Whilst parents were content with the decision made to have their child screened, knowledge about screening, the conditions involved and their potential impact was low. The information given pre‐screening could be improved in terms of consistent provision, the time allocated and the involvement of the midwife.
Leaflets can provide a consistent quality and quantity of information about expanded screening to all parents. Whilst the adapted ‘Newborn blood spot screening for your baby’ leaflet was considered adequate for decision making, differing individual information requirements were evident. More detailed resources are recommended to enable parents to choose from a range of further information available to suit their individual requirements. Autonomous decision making can be addressed through a pick‐and‐mix approach to information provision. Parents should be empowered to choose information relevant to their own decision‐making approach and information processing capabilities. 11 To prevent deterioration in the capacity to make an informed decision, further simplification of the language and medical terminology as well as a readability assessment may be appropriate. 21 It would be appropriate to include parents in the design of the information and its allocation to the standard leaflet or more detailed additional resources.
Timing of information provision
Screening leaflets provided too early in pregnancy were not meaningful and often forgotten, whilst information given soon after birth, or directly before the heel‐prick, also failed to facilitate an informed decision. 30 Information provision late in pregnancy, for example during a late antenatal appointment with a verbal reminder days before the test from the midwife is considered most appropriate. At this time, the information is salient, and an acceptable time period is available to digest it and allow an informed decision.
Role of the midwife
The preference for midwife involvement was clear, and midwife contribution to the screening process could be improved. Trust in and the relationship with the midwife are key to encouraging participation and parental satisfaction with their decision. 21 In terms of an expanded screening programme, midwifery support is needed to ensure consistency and quality in the approach to providing information, assessing understanding and ensuring and supporting decision making.
Willingness to participate in the screening programme
The majority of parents would include their child in an expanded newborn screening programme. The distinction between service development and existing clinical practice within this context however is unclear. For the parent, the interlinked nature may lead to specific ethical issues, for example whether the pre‐scheduled appointment with the midwife for routine screening undermines the option of non‐participation in the expanded screening programme. For midwives, this may give priority to screening, at the expense of informed consent. Furthermore, the uptake of routine screening could be affected by an invitation to take part in expanded screening. These conflicts need further consideration. Clarity is essential, as is the possibility to opt‐out of expanded screening, whilst committing to routine screening.
Obtaining consent
In terms of consent, a system of informed dissent or opting out, in line with current service provision was seen to be desirable by nearly three quarters of participants. However, improvements could be made to the existing informed dissent/choice model with the optional nature of screening being made more transparent. Whilst a formal signature from the parent to document consent may lead to reassurance that information had been adequately relayed and a decision consciously made, it was not deemed necessary by the majority of participants.
Limitations of the research
The mixed methodology employing focus groups and an online survey was designed to capture a range of different perspectives. The aim was to establish as broad a picture as possible, by recruiting both parents and parents‐to‐be from different ethnic backgrounds. The recruitment of fathers and parents of children affected by inherited conditions proved difficult. The context‐specific nature of the subject matter results in it being meaningful within a certain period of life, and as a result, the participants are typical of those considering the screening decision, rather than the wider population.
Experiences reported are based upon memory from a time that the participants themselves recognized as being difficult. Therefore, recollections about information provision for example may be inaccurate. This highlights the challenge in facilitating effective decision making during the first 5–8 days of a child’s life, particularly for first time parents.
Conclusions
It might be argued that service developments require full written informed consent, irrespective of their nature and design. However, there are significant disadvantages to adopting this model in an expansion of newborn screening. 25 Ponder et al. 36 argue that ethical review needs to be more flexible in its attitude to the provision of information and consent processes. Here, consent should not be seen in isolation, but as part of a process where patients are already consenting to have blood taken, and the developmental element just applies to subsequent testing of that blood. A more flexible approach would facilitate the evaluation of new tests whilst ensuring participants are adequately informed of the risk and benefits associated with the project.
Parents need to be provided with comprehensive information about expanded screening at a time that enables them to constructively use this information in decision making. The ‘Newborn blood spot screening for your baby’ leaflet was considered to provide adequate information to allow parents to make an informed decision. However, midwives need to have a consistent role in drawing attention to the leaflet and in providing information based upon parental needs. More detailed resources should be available from which parents can ‘pick and mix’ to empower autonomous decision making.
Gaining informed consent from parents is complex and time consuming. However, consent processes need to be flexible and proportionate with the project being undertaken and with participant expectations. Parental views indicate that a model of informed dissent is adequate for expanded screening.
Sources of funding and conflicts of interest
This work was funded by the Genetics theme of the South Yorkshire Collaboration for Leadership in Applied Health Research and Care, led by Dr J Bonham at Sheffield Children’s (NHS) FT as part of the evaluation of an expanded screening programme in the United Kingdom. This work was to inform the design of parental communication and consent elements of that programme.
Acknowledgements
The researchers would like to thank the participants who took part in this project, as well as the organizations that helped with recruitment including the local children centres, the National Childbirth Trust, Children Living with Inherited Metabolic Diseases (CLIMB), SHS International, Genetic Interest Group, Cystic Fibrosis Trust, OSCAR (Organisation for Sickle Cell Anaemia Research). In the development of the methodology, materials and subsequent review of this work, we would like to thank the expanded newborn screening team. Elizabeth Pottinger is thanked for her support in the data collection and collation. The Coventry University Ethics Committee granted approval for this work. This research was supported by NIHR CLAHRC for South Yorkshire. Further details, including partner details, can be found at http://www.clahrc‐sy.nihr.ac.uk.
References
- 1. UK National Screening Committee . Available at: http://www.screening.nhs.uk, accessed 11 July 2011.
- 2. Levy HL. Newborn screening by tandem mass spectrometry: a new era. Clinical Chemistry, 1998; 44: 2401–2402. [PubMed] [Google Scholar]
- 3. Wilson JMG, Jungner G. Principles and Practice of Screening for Disease. Geneva: World Health Organisation, 1968. [Google Scholar]
- 4. Bijarnia S, Wiley V, Carpenter K, Christodoulou J, Ellaway C, Wilcken B. Glutaric aciduria type I: outcome following detection by newborn screening. Journal of Inherited Metabolic Disease, 2008; 31: 503–507. [DOI] [PubMed] [Google Scholar]
- 5. Vockley J, Ensenauer R. Isovaleric acidemia: new aspects of genetic and phenotypic heterogeneity† American Journal of Medical Genetics Part C: seminars in Medical Genetics. Special Issue: Inborn Errors of Metabolism, 2006; 142C: 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chrastina P, St’astna S, Myskova H, Kosarova M, Elleder M, Zeman J. Newborn screening of inherited metabolic disorders by tandem mass spectrometry. Klinicka Biochemie a Metabolismus, 2005; 13: 77–80. [Google Scholar]
- 7. Autti‐Ramo I, Makela M, Sintonen H et al. Expanding screening for rare metabolic disease in the newborn: an analysis of costs, effect and ethical consequences for decision‐making in Finland. Acta Paediatrica, International Journal of Paediatrics, 2005; 94: 1126–1136. [DOI] [PubMed] [Google Scholar]
- 8. den Boer ME, Wanders RJ, Morris AA, IJlst L, Heymans HS, Wijburg FA. Long‐chain 3‐hydroxyacyl‐CoA dehydrogenase deficiency: clinical presentation and follow‐up of 50 patients. Pediatrics, 2002; 109: 99–104. [DOI] [PubMed] [Google Scholar]
- 9. UK National Screening Committee Second report of the National Screening Committee , 2002. Available at: http://www.isuogmacau2011.com/assets/Uploads/aogm/Guidelines/RCOG‐‐‐UK/Second‐report‐of‐UK‐NSC.pdf, accessed 11 July 2011.
- 10. Medical Research Council Human tissue and biological samples for use in research. Operational and Ethical Guidelines, 2001. Available at: http://www.mrc.ac.uk/Utilities/Documentrecord/index.htm?d=MRC002420, accessed 11 July 2011.
- 11. Bailey DB, Skinner D, Davis AM, Whitmarsh I, Powell C. Ethical, legal, and social concerns about expanded newborn screening: fragile X syndrome as a prototype for emerging issues. Pediatrics, 2008; 121: 693–704. [DOI] [PubMed] [Google Scholar]
- 12. Hargreaves KM, Stewart RJ, Oliver SR. Informed choice and public health screening for children: the case of blood spot screening. Health Expectations, 2005; 8: 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stewart R, Oliver S. What is known about communication with parents about newborn blood spot screening? UK Newborn Screening Programme Centre. London, 2003. Available at: http://newbornbloodspot.screening.nhs.uk/cms.php?folder=2490, accessed 11 July 2011.
- 14. Pollitt RJ, Green A, McCabe A et al. Neonatal screening for inborn errors of metabolism: cost, yield and outcome. Health Technol Assess, 1997; 1: i–iv, 1–202. [PubMed] [Google Scholar]
- 15. Chadwick R, ten Have H, Husted J et al. Genetic screening and ethics: European perspectives. Journal of Medical Philosophy, 1998; 23: 255–273. [DOI] [PubMed] [Google Scholar]
- 16. Stewart F. Ethics of newborn screening. Current Paediatrics, 2006; 16: 216–220. [Google Scholar]
- 17. Kemper AR, Fant KE, Clark SJ. Informing parents about newborn screening. Public Health Nursing, 2005; 22: 332–338. [DOI] [PubMed] [Google Scholar]
- 18. El‐Wakeel H, Taylor GJ, Tate JJT. What do patients really want to know in an informed consent procedure? A questionnaire‐based survey of patients in the Bath area, UK. Journal of Medical Ethics, 2006; 32: 612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Østerlie W, Solbjør M, Skolbekken J‐A, Hofvind S, Sætnan AR, Forsmo S. Challenges of informed choice in organised screening. Journal of Medical Ethics, 2008; 34: e5. [DOI] [PubMed] [Google Scholar]
- 20. Arnold CL, Davis TC, Frempong JO et al. Assessment of newborn screening parent education materials. Pediatrics, 2006; 117 (5 Pt 2): S320–S325. [DOI] [PubMed] [Google Scholar]
- 21. Cooke RW. Good practice in consent. Seminars In Fetal & Neonatal Medicine, 2005; 10: 63–71. [DOI] [PubMed] [Google Scholar]
- 22. Helgesson G, Swartling U. Views on data use, confidentiality and consent in a predictive screening involving children. Journal of Medical Ethics, 2008; 34: 206–209. [DOI] [PubMed] [Google Scholar]
- 23. O’Neill O. Autonomy and Trust in Bioethics. Cambridge: Cambridge University Press, 2002. [Google Scholar]
- 24. Helgesson G, Ludvigsson J, Gustafsson Stolt U. How to handle informed consent in longitudinal studies when participants have a limited understanding of the study. Journal of Medical Ethics, 2005; 31: 670–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tarini BA, Wylie Burke W, Scott RC, Wilfon BS. Waiving informed consent in newborn screening research: Balancing social value and respect. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 2008; 148C: 22–30. [DOI] [PubMed] [Google Scholar]
- 26. Junghans C, Feder G, Harry Hemingway H, Timmis A, Jones M. Recruiting patients to medical research: blind randomised trial of “opt‐in” versus double “opt‐out” strategies. British Medical Journal, 2005; 331: 940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rogers CG, Tyson E, Kennedy KA, Broyles RS, Hickman JF. Conventional consent with opting in versus simplified consent with opting out: an exploratory trial for studies that do not increase patient risk. Journal of Pediatrics, 1998; 132: 606–611. [DOI] [PubMed] [Google Scholar]
- 28. Liebl B, Nennstiel‐Ratzel U, von Kries R et al. Very high compliance in an expanded MS‐MS‐based newborn screening program despite written parental consent. Preventative Medicine, 2002; 34: 127–131. [DOI] [PubMed] [Google Scholar]
- 29. Davis TC, Humiston SG, Arnold CL et al. Recommendations for effective newborn screening communication: results of focus groups with parents, providers, and experts. Pediatrics, 2006; 117 (5 Pt 2): S326–S340. [DOI] [PubMed] [Google Scholar]
- 30. Davey A, French D, Dawkins H, O’Leary P. New mothers’ awareness of newborn screening, and their attitudes to the retention and use of screening samples for research purposes. Genomics, Society and Policy, 2005; 1: 41–51. [Google Scholar]
- 31. Detmar S, Hosli E, Dijkstra N, Nijsingh N, Rijnders M, Verweij M. Information and Informed Consent for Neonatal Screening: opinions and Preferences of Parents. Birth, 2007; 34: 238–244. [DOI] [PubMed] [Google Scholar]
- 32. Detmar S, Dijkstra N, Nijsingh N, Rijnders M, Verweij M, Hosli E. Parental Opinions about the Expansion of the Neonatal Screening Programme. Community Genetics, 2008; 11: 11–17. [DOI] [PubMed] [Google Scholar]
- 33. SurveyMonkey.com . http://www.surveymonkey.com, accessed 11 July 2011.
- 34. UK Newborn Screening Programme Centre Newborn blood spot screening for your baby. Available at: http://newbornbloodspot.screening.nhs.uk/languages, accessed 11 July 2011.
- 35. Braun V, Clarke V. Using thematic analysis in psychology. Qualitative Research in Psychology, 2006; 3: 77–101. [Google Scholar]
- 36. Ponder M, Statham H, Hallowell N, Moon JA, Richards M, Raymond FL. Genetic research on rare familial disorders: consent and the blurred boundaries between clinical service and research. Journal of Medical Ethics, 2008; 34: 690–694. [DOI] [PMC free article] [PubMed] [Google Scholar]


