Table 3.
Oxidation of styrenyl substrates with the Pd(NHC)–TBHP system.59
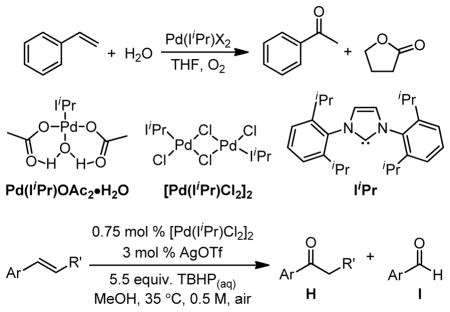
| |||||
|---|---|---|---|---|---|
| Ar | R′ | time (h) | % conversion | % yield | H:I |
| Ph | H | 24 | >99 | 75 | >130:1 |
| 2-MePh | H | 48 | >99 | 79 | 36:1 |
| 3-MePh | H | 32 | >99 | 83 | 22:1 |
| 4-MePh | H | 16 | >99 | 86 | 22:1 |
| 2,4,6-MePh | H | 24 | 95 | 71 | >150:1 |
| 3-CIPh | H | 48 | >98 | 80 | >150:1 |
| 3-NO2Pha | H | 24 | 90 | 79 | >150:1 |
| Phb | Ph | 48 | NA | 42c | 42:35 |
| Ph | Me | 48 | 97 | NA | 2.3:1d |
Conditions: 2.25 mol % [Pd(IiPr)Cl2]2, 12 mol % AgOTf.
Conditions: [alkene] = 0.3 M, 1.25 mol % [Pd(IiPr)Cl2]2, 4 mol % AgOTf, 35–50 °C.
Two equivalents of benzaldehyde produced via oxidative cleavage.
H is a 53:47 mixture of regioisomeric ketones.
