Abstract
An aggregation of moxifloxacin-resistant Clostridium difficile ribotype 231 (RT231) isolates was first identified in the county of Stockholm in 2008, and by the end of 2015 isolates of RT231 had spread to 13 of 21 Swedish counties. We investigated the epidemiology of C. difficile RT231 in Sweden between 2006 and 2015 using whole genome sequencing (WGS) and evaluated whether its emergence could be associated with extended moxifloxacin use. We performed WGS and phylogenetic analysis of 51 C. difficile RT231 strains isolated in Sweden over a 10-year period. We also calculated the county-specific prescription rates for moxifloxacin between 2005 and 2015. Using WGS and detailed single nucleotide polymorphism analysis, we demonstrated three divergent sublineages of moxifloxacin-resistant C. difficile RT231 in Sweden from 2008 to 2015. A set of closely related RT231 was identified in hospitals located in the counties of Stockholm and Uppsala in 2008. Another set of RT231 isolates was found in four different counties in the Uppsala–Örebro Health Care Region. A gradual drop in moxifloxacin use in the county of Stockholm coincided with a reduction of RT231 in the area. However, RT231 continued to be frequent in surrounding counties including Uppsala, a county that also had the highest moxifloxacin prescription rates. We demonstrated frequent transmission of C. difficile RT231 within and between counties, indicating the importance of careful monitoring of hospitalized individuals infected with moxifloxacin-resistant C. difficile as well as the need for a strict moxifloxacin prescription policy.
Keywords: Clostridium difficile, epidemiology, moxifloxacin consumption, ribotype 231, whole genome sequencing
Introduction
Clostridium difficile, a Gram-positive and spore-forming anaerobic bacillus, is a leading cause of healthcare-associated diarrhoea in industrialized countries [1]. Transmission occurs primarily in healthcare facilities, where exposure to antibiotics and environmental contamination by C. difficile spores are common. Antibiotic treatment, advanced age (>65 years), comorbidities and hospitalization have been identified as major risk factors for C. difficile infection (CDI) [2]. However, antimicrobial therapy is the single most important risk factor for CDI. It leads to the disruption of the normal intestinal flora, creating favourable conditions for acquisition and proliferation of C. difficile [3]. Clindamycin, amoxicillin, third-generation cephalosporins and more recently fluoroquinolones have been shown to be associated with a particularly high risk of developing CDI [4].
The first whole genome sequence of C. difficile was obtained in 2006; sequencing revealed a large circular chromosome of 4 290 252 bp likely coding for 3776 proteins and an extracircular plasmid 7881 bp in size [5]. Several other complete or almost complete genomic sequences have since been obtained [6]. These studies have shown that the C. difficile genome contains a high proportion (over 10%) of mobile genetic elements including bacteriophages, repeat elements and genomic islands, as well as transposable and conjugative elements.
Several typing methods have been used to study the epidemiology, genetic diversity and evolution of C. difficile. In Europe PCR ribotyping has been one of the most widely adopted methods, and over 600 C. difficile ribotypes have been identified. A particular subtype, C. difficile ribotype 027, has been associated with severe disease and hospital outbreaks in North America and Europe [7], [8], [9]. Global emergence of this moxifloxacin-resistant C. difficile type 027 prompted establishment of national surveillance programs in several European countries [10], [11], [12]. As a result of national surveillance, a clonal aggregation of a newly emerging, moxifloxacin-resistant C. difficile ribotype 231 (RT231) was uncovered in Sweden in 2008 [12]. This type was initially geographically limited to the Stockholm area, where over 70% of all moxifloxacin-resistant C. difficile strains were characterized as RT231 in 2008 (125/144) [12]. Despite an increasing awareness of CDI and enhanced infection control policies, RT231 has continued to spread in Sweden; by the end of the year 2015 it had been identified in 13 of 21 counties, suggesting interhospital transmission and possible local outbreaks [13].
Although no studies have shown whether moxifloxacin use implies higher risk for CDI than other commonly used antibiotics [14], the restriction of moxifloxacin use has been associated with a reduction in the number of CDIs in Northern Ireland and England [15], [16]. Similarly, a study from Vienna demonstrated that enhanced antibiotic stewardship, including restriction of moxifloxacin use, was associated with a reduction in the number of CDI in population with a high rate of multidrug-resistant C. difficile [17].
We used whole genome sequencing (WGS) and phylogenetic analysis to investigate the spread of this newly emerging moxifloxacin-resistant C. difficile RT231 in Sweden between 2006 and 2015. We also evaluated the potential association between the extent of moxifloxacin prescription rates and the epidemiology of RT231.
Materials and Methods
Collection of C. difficile isolates
All 189 C. difficile RT231 isolates identified in Sweden between 2006 and 2015 were included in this study (Table 1). The strains collected between 2009 and 2015 were obtained through the national surveillance program for C. difficile; before 2009 samples were obtained via a nationwide surveillance study [12]. Date of sample and county of diagnostic laboratory were collected as previously described [12]. PCR ribotyping between 2006 and 2012 was performed by a gel-based method [12] and between 2013 and 2015 by capillary-gel electrophoresis [18]. Fifty-one of 189 C. difficile RT231 isolates were selected for WGS. All moxifloxacin-sensitive strains were included, and moxifloxacin-resistant isolates were selected to cover each isolation region per year to obtain a geographically and temporally representative sample collection. The number of patients or cases of CDI was not available.
Table 1.
Total number of Clostridium difficile isolates collected in Sweden between 2006 and 2015, and numbers of C. difficile ribotype 231 (RT231) isolates included in study
| Year | All C. difficile isolates | Moxifloxacin-resistant isolates | Moxifloxacin-resistant RT231 | Moxifloxacin-sensitive RT231 | RT231 isolates included in study (frequency of strains included per year) |
|---|---|---|---|---|---|
| 2006 | 13 | 1 | 1 | Unknowna | 1 (1/1) |
| 2007 | 22 | 6 | 6 | Unknowna | 1 (1/6) |
| 2008 | 586 | 586 | 125 | Unknowna | 11 (11/125) |
| 2009 | 393 | 57 | 8 | 1 | 6 (6/9) |
| 2010 | 335 | 53 | 5 | 1 | 6 (6/6) |
| 2011 | 418 | 59 | 8 | 0 | 6 (6/8) |
| 2012 | 423 | 87 | 16 | 1 | 7 (7/17) |
| 2013 | 459 | 60 | 8 | 0 | 4 (4/8) |
| 2014 | 391 | 57 | 6 | 0 | 6 (6/6) |
| 2015 | 413 | 43 | 3 | 0 | 3 (3/3) |
| Total | 3446 | 1002 | 186 | 3 | 51 (51/189) |
In 2006 C. difficile typing was performed only for diagnostic purposes, and in 2007–2008 only moxifloxacin-resistant C. difficile strains were collected.
Antimicrobial susceptibility assays
Isolates were grown on Mueller-Hinton fastidious agar, and minimum inhibitory concentration (MIC) values for moxifloxacin, erythromycin, clindamycin, metronidazole and vancomycin were determined using Etests (bioMérieux, Marcy l’Etoile, France) as described previously [12]. The epidemiologic cutoff breakpoints for resistance were as follows: metronidazole MIC >2 mg/L, vancomycin MIC >2 mg/L, erythromycin MIC >2 mg/L, clindamycin MIC >16 mg/L and moxifloxacin MIC >4 mg/L, according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST).
Isolation and DNA extraction
Isolates were recultured on blood agar plates, inoculated into sterile peptone yeast liquid medium with 0.5 mg/mL cysteine and grown overnight anaerobically at 35°C. Cells were pelleted, suspended into lysis buffer (1 mM Tris, 0.1 mM EDTA, 0.1% Triton-X and 20 mg/mL lysozyme) with proteinase K (180 μL of buffer and 20 μL of proteinase K (20 μL/mL)) and incubated for 18 hours at 56°C. Genomic DNA was extracted using MagAttract DNA Mini M48 Kit and Biorobot M48 according to the manufacturer's recommendations (Qiagen, Sollentuna, Sweden). DNA was eluted into a final volume of 100 μL.
Whole genome sequencing
WGS was performed using the Ion Torrent platform, aiming to read lengths of 200 bp and coverage of 30×. The sequence data were analysed by CLC Assembly Cell 4.4.2 software. Sequence type (ST) was determined for comparison using mapping and the Basic Local Alignment Search Tool (BLAST) against the C. difficile multilocus sequence typing (MLST) database (http://pubmlst.org/cdifficile/). Strain MX213 was used as the reference genome for single nucleotide polymorphism (SNP) analysis. The raw data were mapped to the reference genome, and variants were called. Positions with low coverage (<10) or ambiguous calls (<90%) in one or more samples were filtered out. SNPs within 500 bp of each other were filtered out as single events from the SNPs in the phylogenetic analysis to remove nonrandomly distributed mutations; these are collectively termed recombination events. These mutations are considered a single event for the purpose of genetic distance. A minimum spanning tree was constructed on the basis of the all SNP differences. The analysis was repeated excluding the four nonrelated strains; the remaining individual SNP positions were compiled into an alignment and used for phylogenetic analysis.
Phylogenetic analysis
Neighbour-joining phylogenetic trees were constructed on the basis of the SNP alignments using MEGA 6.0 and 100 bootstrap replicates. Metadata including year of isolation and geographical location were transferred to the reconstructed tree. The genomic relatedness of isolates was assessed by SNP analysis: up to three individual SNPs per genome was expected to be seen between related isolates collected up to 1 year apart, whereas strains which differed more than ten individual SNPs were classified as unrelated.
Antibiotic data
Data on moxifloxacin prescriptions were obtained from Apotekens Service AB (2003–2013) and from the Swedish eHealth Agency (2014–2015). The data include moxifloxacin prescribed to patients as well as sales to hospitals and other healthcare providers.
Results
Whole genome sequencing
A total of 51 of 189 C. difficile RT231 isolates selected for WGS were geographically and temporally representative; they included isolates from all 13 counties where RT231 strains had been isolated over a 10-year period (Table 1, Table 2). An average minimum coverage of 60.07 (range 20.52–120.13) reads per nucleotide and an average genome length of 4.14 (range 3.92–4.30) Mbp were obtained for all samples.
Table 2.
Total number of Clostridium difficile ribotype 231 (RT231) isolates collected in each healthcare region and county in Sweden between 2006 and 2015
| Healthcare region | County | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stockholm | Stockholm | 1 | 6 | 119 | 5 | 1 | 2 | 1 | 135 | |||
| Gotland | 3 | 3 | ||||||||||
| Uppsala–Örebro | Uppsala | 4 | 1 | 4 | 6 | 1 | 1 | 17 | ||||
| Södermanland | 1 | 5 | 6 | 3 | 1 | 16 | ||||||
| Västmanland | 2 | 4 | 1 | 1 | 8 | |||||||
| Dalarna | 1 | 1 | ||||||||||
| Örebro | 1 | 1 | 2 | |||||||||
| Värmland | 1 | 1 | ||||||||||
| Gävleborg | ||||||||||||
| North | Norrbotten | 1b | 1 | |||||||||
| Västerbotten | ||||||||||||
| Jämtland | ||||||||||||
| Västernorrland | ||||||||||||
| Southeast | Östergotland | |||||||||||
| Jönköping | 1a | 1 | ||||||||||
| Kalmar | ||||||||||||
| West | Västra Götaland | 1a | 1 | |||||||||
| Halland (partly) | ||||||||||||
| South | Kronoberg | |||||||||||
| Blekinge | 2 | 2 | ||||||||||
| Skåne | 1a | 1 | ||||||||||
| Halland (partly) | ||||||||||||
| Total | 1 | 6 | 125 | 9 | 6 | 8 | 17 | 8 | 6 | 3 | 189 |
These C. difficile RT231 isolates are moxifloxacin sensitive.
This isolate was shown not to be PCR RT231.
No previous WGS data were available for C. difficile RT231, and thus the first whole genome sequence of RT231 was obtained by a de novo assembly of strain MOX213 (3.92 Mbp). This was used as a reference genome in further analysis. MLST data obtained from whole genome sequence representing approximately 0.09% of the whole genome (3501 nt) were almost consistent with ribotyping data; all studied isolates belonged to ST133. One isolate (NT949), determined to be PCR RT231 by a gel-based method, belonged to ST133, but PCR ribotyping analysis by capillary-gel electrophoresis with higher resolution suggested a new, unknown ribotype.
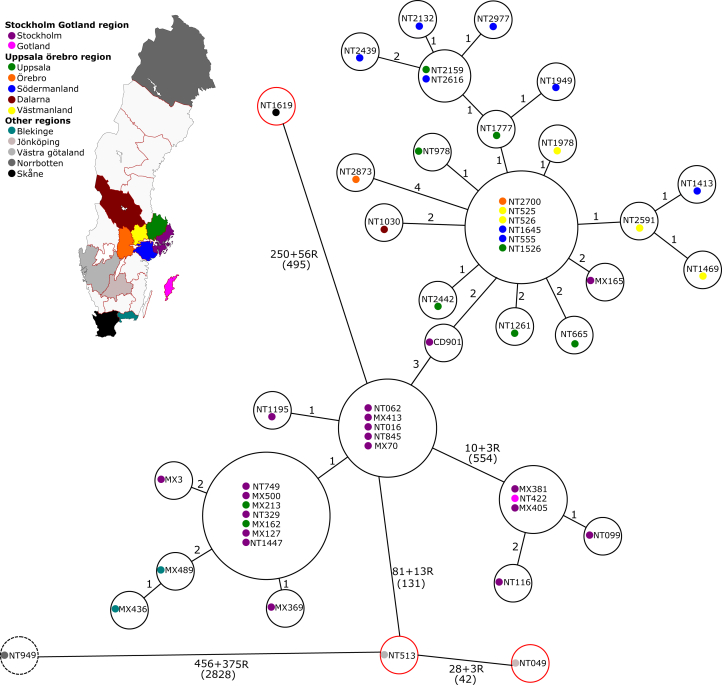
A total of 4091 nucleotide differences were identified within the 51 isolates; 3225 nucleotide differences were classified as recombination events, whereas the remaining 866 nucleotide differences were classed as individual SNPs. The results are presented in a minimal spanning tree in Fig. 1 (88.56% of the genome was used as the dynamic core genome). A total of 3496 nucleotide differences distinguished four single isolates (three moxifloxacin-sensitive isolates NT049/Jönköping_2009, NT513/VästraGötaland_2010 and NT1619/Skåne_2012 and one moxifloxacin-resistant isolate NT949/Norrbotten_2011) from the remaining 47 isolates (all moxifloxacin-resistant isolates). A total of 2681 nucleotide differences out of 3496 were filtered as 447 recombination events. Within the remaining 47 isolates, five strains (MX405/Stockholm_2008, MX381/Stockholm_2008, NT422/Gotland_2009, NT116/Stockholm_2009 and NT099/Stockholm_2009) differed by 554 nucleotides from the closest main cluster of 42 isolates. A total of 544 nucleotide differences out of 554 were filtered as three recombination events.
Fig. 1.
Minimum spanning tree of Clostridium difficile RT231 isolates included in study. Isolates in red bubbles are moxifloxacin sensitive; isolate in dashed bubble belongs to ST131 but not RT231. SNP differences are shown next to branches, recombination events are marked R, and total number of nucleotide differences is shown in parentheses. Length of branches is not relative to relationship distance.
Phylogenetic analysis
In order to study potential transmission of moxifloxacin-resistant C. difficile RT231 between different counties during the 10-year study period, a phylogenetic comparison of 47 strains based on the core genome SNP analysis was performed (excluding the unrelated isolates NT049, NT513, NT949 and NT1619). A total of 93.2% of the genome was used in the analysis as the dynamic core genome. A total of 566 nucleotide differences were identified, of which 510 were classified as recombination events. The remaining 56 phylogenetically representative individual SNPs were included in the phylogenetic analysis. SNP differences between isolates were small, varying from zero to 11 SNPs.
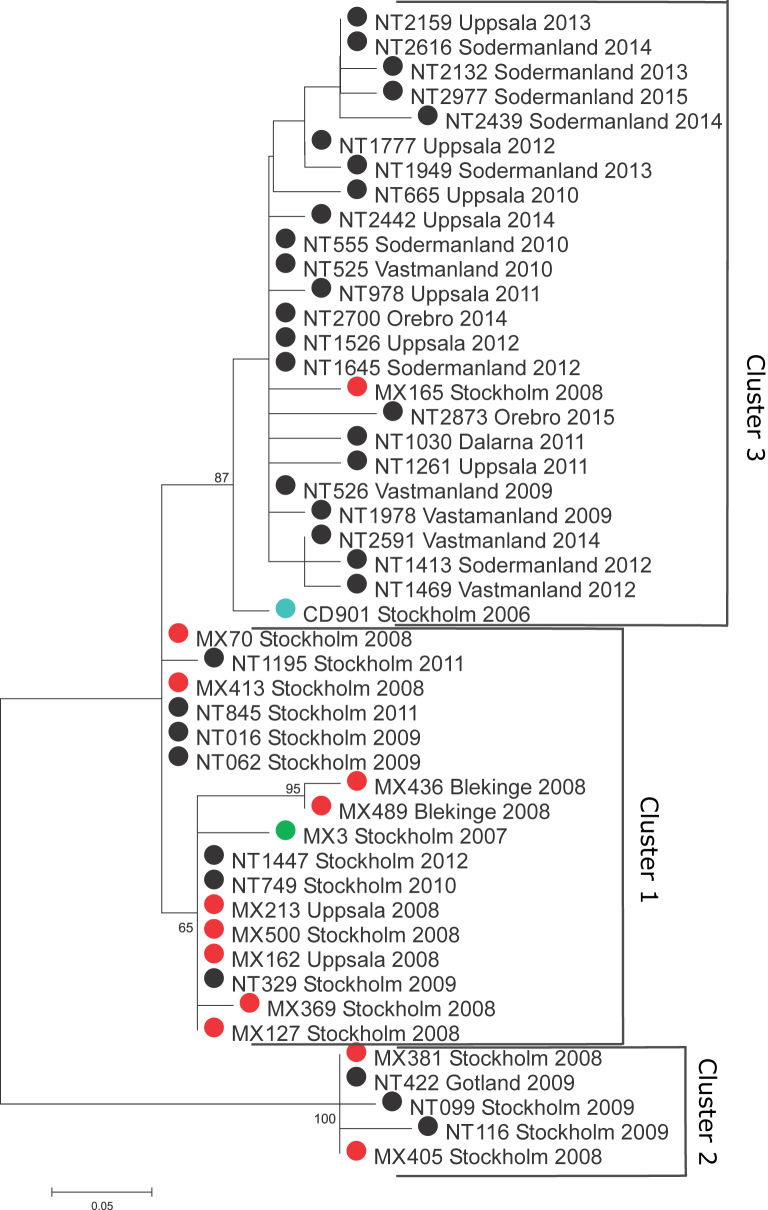
Three main clusters could be identified. Seventeen isolates collected between 2007 and 2012 in the counties of Stockholm, Uppsala and Blekinge formed the first cluster, with only 3 SNPs' difference between them (cluster 1, Fig. 2). A clonal strain (defined as ≤1 SNP difference) was identified in five different hospitals in the counties of Stockholm and Uppsala during 2008 and 2009. The remaining Stockholm isolates from 2008 and 2009 (n = 4; all from the same hospital) formed their own bootstrap supported cluster together with a single isolate identified in Gotland during the same time period (cluster 2, Fig. 2). The third cluster was composed of the oldest isolate identified in Stockholm in 2006, another Stockholm isolate from 2008 and all the other isolates collected in Uppsala (n = 7), Västmanland (n = 5), Södermanland (n = 8), Örebro (n = 2) and Dalarna (n = 1), with a maximum difference of 3 SNPs between the isolates (cluster 3, Fig. 2). A second clonal strain was circulating in the counties of Uppsala, Södermanland, Västmanland and Örebro between 2009 and 2015.
Fig. 2.
Neighbour-joining tree of Clostridium difficile RT231 isolates circulating in Sweden between 2006 and 2015. Samples are colour coded according to year of isolation: 2006 (blue), 2007 (green), 2008 (red) and 2009 to 2015 (black).
Antimicrobial resistance
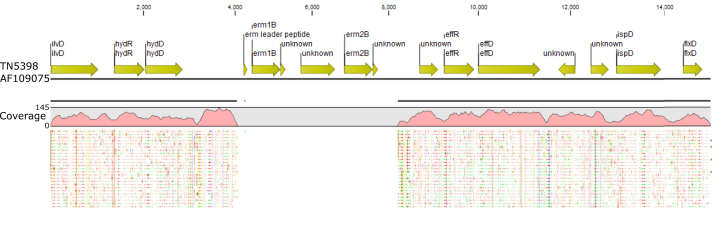
In total, 48 of the 51 C. difficile RT231 isolates were resistant to moxifloxacin according to EUCAST epidemiologic cutoff MIC breakpoints. All moxifloxacin-resistant C. difficile RT231 isolates identified between 2009 and 2015 were also resistant to clindamycin and erythromycin. No isolate was resistant to metronidazole or vancomycin. All moxifloxacin-resistant strains harboured an amino acid substitution in the gyrA gene (Thr82 > Ile); the unrelated moxifloxacin-resistant NT949 strain had an additional amino acid substitution in the gyrB gene (Asp426 > Asn). No other mutations within the GyrA or GyrB genes were identified. We screened the isolates for the presence of the ermB gene known to confer macrolide and lincosamide resistance. Interestingly all isolates carried most of the TN5398 transposon with a deletion of the ermB locus (Fig. 3). All isolates were also negative for the other predominant erm genes found in anaerobes (A, C, E, F and Q) [19]. Both sensitive and resistant strains carried the cme gene, which encodes an efflux pump similar to mefA, shown to confer erythromycin resistance [20]. Furthermore, the 23S rRNA sequences were analysed, but no differences were found between sensitive and resistant strains. A mutation in the rplD gene (G211A, Ala71 > Thr), encoding the L4 protein of the 50S ribosomal subunit, was found in 25 of 47 resistant isolates, with all isolates belonging to cluster 3. All five isolates in cluster 2, on the other hand, carried a mutation in the rplV gene (G313A, Val105 > Ile) encoding the L22 protein of the 50S ribosomal subunit.
Fig. 3.
Deletion of ermB gene locus in Clostridium difficile RT231 isolate MX70 mapped against TN5398 transposon.
Prescription of moxifloxacin in Sweden between 2005 and 2015
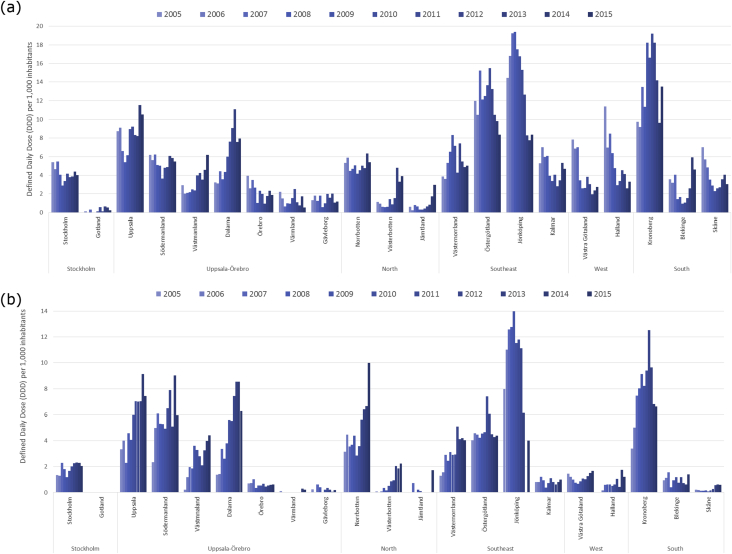
To evaluate a possible link between moxifloxacin consumption and the spread of RT231, we analysed the county-specific consumption rates for moxifloxacin. The mean community prescription rate of moxifloxacin in Sweden decreased from 9.2 defined daily doses (DDD) per 1000 inhabitants per year in 2003 to 4.46 in 2015, whereas the mean hospital prescription rates increased from 1.17 DDD per 1000 inhabitants per year in 2003 to 2.59 in 2015. The differences in the community moxifloxacin prescription between counties became smaller over time (range in community 0.24–23.26 (2003) and 0.54–11.53 (2014) DDD per 1000 inhabitants per year), whereas the opposite was seen in the hospital prescription rates between counties (range in hospital 0.13–3.25 (2003) and 0.18–9.13 (2014) DDD per 1000 inhabitants per year). In 2014 the highest prescription rates, both in community and hospital settings, were identified in Uppsala county (11.53 and 9.13 DDD per 1000 inhabitants per year, respectively) (Fig. 4). Hospital use of moxifloxacin was also high in Södermanland county in 2014 (9.02 DDD per 1000 inhabitants per year). Two other counties, Jönköping and Kronoberg, were noted also to have had high overall prescription rates of moxifloxacin in both community and hospital settings.
Fig. 4.
Prescription of moxifloxacin in community (a) and hospital (b) settings by county in Sweden between 2005 and 2015. Prescription data in hospital setting is incomplete for Jönköping, 2013–2015.
Discussion
We investigated the emergence and epidemiology of moxifloxacin-resistant C. difficile RT231 in Sweden between 2006 and 2015 by WGS and compared the geographical spread of this moxifloxacin-resistant type to county-specific moxifloxacin prescription rates. A majority of RT231 isolates identified in Sweden since 2006 have been moxifloxacin resistant (98%, 186/189). The proportion of moxifloxacin resistance among all C. difficile isolates has been around 15% in Sweden between 2009 and 2015 (range, 10–21%), which is less than previously reported: 40% in Europe [21]. The amino acid substitution (Thr82 > Ile in gyrA gene) is the most frequently described mutation associated with fluoroquinolone-resistant C. difficile, including the hypervirulent ribotype 027 [20]; it was also present in all moxifloxacin-resistant RT231 isolates studied. Although association between multiple substitutions and high-level moxifloxacin resistance have been previously demonstrated [22], all resistant isolates of RT231 included in our study harboured only a single mutation and showed high-level resistance (MIC >32 mg/L). Interestingly, most of the TN5398 transposon was detected, but with a deletion of the ermB gene locus, known to confer macrolide and lincosamide resistance [23]. No mutations were found in the 23S rRNA sequence. Macrolide and lincosamide resistance is thus most likely conferred by an as-yet unknown mechanism. Although isolates belonging to clusters 2 and 3 carried mutations in the L4 or L22 proteins of the 50S ribosomal subunit—proteins associated with macrolide resistance [24], [25]—the closely related isolates in cluster 1 have L4 and L22 proteins identical to the erythromycin-sensitive strains, and we believe that an evolution of separate resistance mechanisms is unlikely.
Although C. difficile RT231 was first identified in a Stockholm hospital as early as in 1999, it remained a rare type until the outbreak was identified in Stockholm county in 2008 [12], [26]. On the basis of the previously estimated evolutionary rate of 0.74 SNP per year for C. difficile, up to 3 SNPs per genome would be expected to be seen between related isolates collected up to 365 days apart [27]. By using these previous estimates of C. difficile evolution, we concluded that the first two RT231 strains are likely related and originated from a common ancestor. These isolates were isolated almost by a 2-year gap in Stockholm (CD901, cluster 2; MX3, cluster 1) and harbour 6 SNP differences in between. In contrast, two genetically distinct C. difficile genotypes were also shown to be circulating in Stockholm in 2008, as demonstrated by more than 10 SNPs' difference between the isolates (MX70 and MX381). Interestingly, a third genetically distinct C. difficile genotype (MX165) differing from MX70 by five nucleotides was also identified in Stockholm during the same year. This shows that at least two divergent sublineages of moxifloxacin-resistant C. difficile RT231 were already circulating in the Stockholm area when the outbreak was found in 2008, and other strains related to these outbreak strains had likely been circulating in the area years before that. The continued circulation of a clonal strain, demonstrated both in the Stockholm and Uppsala–Örebro healthcare regions, suggests that RT231 strains can persist for a long time in healthcare settings. Because the spread of clonal strains (defined as ≤1 SNP difference) occurred mainly within a healthcare region, patient movement through hospitals with specialized care is presumably the main transmission route for spreading C. difficile RT231. It is therefore important to implement strict cleaning procedures and isolation routines for all patients infected with moxifloxacin-resistant strains to stop further transmission.
Spread of RT231 was noted in Stockholm county in 2008, but the emergence had likely already occurred few years before that. It is unclear why it emerged particularly in the Stockholm area, where the prescription rate of moxifloxacin has not been the highest in Sweden. However, it is important to note that the reduced use of moxifloxacin in Stockholm county has coincided with a reduction in the spread of RT231 in the area and the increased moxifloxacin use in Uppsala county with the increased spread of this moxifloxacin-resistant type. Similarly, the counties of Jönköping and Kronoberg, which had high prescription rates of moxifloxacin between 2005 and 2010, were affected by outbreaks of moxifloxacin-resistant C. difficile other than RT231: RT046 and RT027 respectively [13], [28]. In the county of Jönköping, as prescription rates dropped and infection control was implemented, RT046 has virtually been eliminated from the area [28].
Interestingly, in a case–control study in Stockholm county, fluoroquinolones were identified as a statistically significant risk factor for acquiring RT231 CDI, but only a small proportion of individuals infected with this strain had actually received moxifloxacin (Zomer et al., unpublished data). This is keeping with the idea that general antibiotic pressure in the community plays a major role in driving the emergence of resistant strains of C. difficile, and, as in the case of moxifloxacin-resistant RT231, further use of any fluoroquinolone will likely lead to CDI problems in infected individuals [29]. A qualitative goal for limiting fluoroquinolone prescribing in outpatient care in Sweden was set in 2007, but none of the counties has reached the target set (https://www.folkhalsomyndigheten.se/pagefiles/24127/Swedres-Svarm-2015-15099.pdf).
A limitation of this study is the lack of information regarding the number of cases and patient hospitalizations (i.e. a move to another hospital). This study may also contain multiple isolates from the same individuals, so the real number of cases cannot be determined. In addition, because routine surveillance was established in Sweden in 2009, isolates collected before 2009 were not systematically collected. As a further limitation, age-specific moxifloxacin and general fluoroquinolone consumption rates were unavailable for study.
In conclusion, we used SNP analysis based on WGS to investigate the emergence and spread of moxifloxacin-resistant C. difficile RT231 in Sweden over a 10-year study period. We demonstrated the continued transmission of RT231 within and between different close-proximity counties. The outbreak emerged first in the Stockholm area and spread to surrounding counties in the Uppsala–Örebro healthcare region. Our results support the need for careful monitoring of hospitalized individuals infected with moxifloxacin-resistant C. difficile types as well as for stricter limits on fluoroquinolone prescriptions in Sweden. Although RT231 has only been described in Sweden so far, it can potentially spread to countries where other moxifloxacin-resistant C. difficile types have also recently emerged. The factors associated with the emergence of new ribotypes within a certain country remain largely unexplored.
Acknowledgements
We acknowledge all the clinical microbiologic laboratories participating to the Swedish C. difficile surveillance program for their contribution of isolates. We also acknowledge I. Alefjord and J. Furuskog for excellent technical laboratory assistance, and A. Jasir, European Centre for Disease Control, for reviewing early versions of the article.
Conflict of Interest
None declared.
References
- 1.Rupnik M., Wilcox M.H., Gerding D.N. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526–536. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- 2.Kelly C.P., LaMont J.T. Clostridium difficile—more difficult than ever. N Engl J Med. 2008;359:1932–1940. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 3.Barlett J.G. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med. 2002;346:334–339. doi: 10.1056/NEJMcp011603. [DOI] [PubMed] [Google Scholar]
- 4.Gerding D.N. Clindamycin, cephalosporins, fluoroquinolones and Clostridium difficile–associated diarrhoea: this is an antimicrobial resistance problem. Clin Infect Dis. 2004;38:646–648. doi: 10.1086/382084. [DOI] [PubMed] [Google Scholar]
- 5.Sebaihia M., Wren B.W., Mullany P., Fairweather N.F., Minton N., Stabler R. The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nat Genet. 2006;38:779–786. doi: 10.1038/ng1830. [DOI] [PubMed] [Google Scholar]
- 6.Knight D.R., Giglio S., Huntington P.G., Korman T., Kotsanas D., Moore C.V. Surveillance for antimicrobial resistance in Australian isolates of Clostridium difficile, 2013–14. J Antimirob Chemother. 2015;70:2992–2999. doi: 10.1093/jac/dkv220. [DOI] [PubMed] [Google Scholar]
- 7.McDonald L.C., Killgore G.E., Thompson A., Owens R.C., Jr., Kazakova S.V., Sambol S.P. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353:2433–2441. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 8.Loo V.G., Poirier L., Miller M.A., Oughton M., Libman M.D., Michaud S. A predominantly clonal multi-institutional outbreak of Clostridium difficile–associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353:2442–2449. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 9.Kuijper E.J., Barbut F., Brazier J.S., Kleinkauf N., Eckmanss T., Lambert M.L. Update of Clostridium difficile infection due to PCR ribotype 027 in Europe, 2008. Euro Surveill. 2008;13:18942. [PubMed] [Google Scholar]
- 10.Lyytikäinen O., Mentula S., Kononen E., Kotila S., Tarkka E., Anttila V.J. First isolation and report of cluters of Clostridium diffile PCR ribotype 027 in Finland. Euro Surveill. 2007;12:3303. doi: 10.2807/esw.12.45.03303-en. [DOI] [PubMed] [Google Scholar]
- 11.Bacci S., St-Martin G., Olesen B., Bruun B., Olsen K.E., Nielsen E.M. Outbreak of Clostridium difficile 027 in North Zealand, Denmark, 2008–2009. Euro Surveill. 2009;14:19183. [PubMed] [Google Scholar]
- 12.Åkerlund T., Alefjord I., Dohnhammar U., Struwe J., Noren T., Tegmark-Wisell K., Swedish C. difficile Study Group Geographical clustering of cases of infection with moxifloxacin-resistant Clostridium difficile PCR-ribotypes 012, 017 and 046 in Sweden, 2008 and 2009. Euro Surveill. 2011;16:19813. doi: 10.2807/ese.16.10.19813-en. [DOI] [PubMed] [Google Scholar]
- 13.Public Health Agency of Sweden . Public Health Agency of Sweden; Solna, Sweden: 2015. Annual Clostridium difficile report, 2014. [Google Scholar]
- 14.Deshpande A., Pant C., Jain A., Fraser T.G., Rolston D.D. Do fluoroquinolones predispose patients to Clostridium difficile associated disease? A review of the evidence. Curr Med Res Opin. 2008;24:329–333. doi: 10.1185/030079908x253735. [DOI] [PubMed] [Google Scholar]
- 15.Aldeyab M.A., Kearney M.P., Scott M.G., Aldiab M.A., Alahmadi Y.M., Darwish Elhajji F.W. An evaluation of the impact of antibiotic stewardship on reducing the use of high-risk antibiotics and its effect on the incidence of Clostridium difficile infection in hospital settings. J Antimicrob Chemother. 2012;67:2988–2996. doi: 10.1093/jac/dks330. [DOI] [PubMed] [Google Scholar]
- 16.Talpaert M.J., Gopal Rao G., Cooper B.S., Wade P. Impact of guidelines and enhanced antibiotic stewardship on reducing broad-spectrum antibiotic usage and its effect on incidence of Clostridium difficile infection. J Antimicrob Chemother. 2011;66:2168–2174. doi: 10.1093/jac/dkr253. [DOI] [PubMed] [Google Scholar]
- 17.Wenisch J.M., Equiluz-Bruck S., Fudel M., Reiter I., Schmid A., Singer E. Decreasing Clostridium difficile infections by an antimicrobial stewardship program that reduces moxifloxacin use. Antimicrob Agents Chemother. 2014;58:5079–5083. doi: 10.1128/AAC.03006-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rizzardi K., Åkerlund T. High molecular weight typing with MALDI-TOF MS—a novel method for rapid typing of Clostridium difficile. PLoS One. 2015;10(4):e0122457. doi: 10.1371/journal.pone.0122457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spigaglia P., Barbanti F., Mastrantonio P., European Study Group on Clostridium difficile (ESGCD) Multidrug resistance in European Clostridium difficile clinical isolates. J Antimicrob Chemother. 2011;66:2227–2234. doi: 10.1093/jac/dkr292. [DOI] [PubMed] [Google Scholar]
- 20.Lebel S., Bouttier S., Lambert T. The cme gene of Clostridium difficile confers multidrug resistance in Enterococcus faecalis. FEMS Microbiol Lett. 2004;238:93–100. doi: 10.1016/j.femsle.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 21.Freeman J., Vernon J., Morris K., Nicholson S., Todhunter S., Longshaw C., Pan-European Longitudinal Surveillance of Antibiotic Resistance among Prevalent Clostridium difficile Ribotypes’ Study Group Pan-European longitudinal surveillance of antibiotic resistance among prevalent Clostridium difficile ribotypes. Clin Microbiol Infect. 2015;21 doi: 10.1016/j.cmi.2017.10.008. 248.e9–248. [DOI] [PubMed] [Google Scholar]
- 22.Spigaglia P., Carattoli A., Barbanti F., Mastrantonio P. Detection of gyrA and gyrB mutations in Clostridium difficile isolates by real-time PCR. Mol Cell Probes. 2010;24:61–67. doi: 10.1016/j.mcp.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Mullany P., Wilks M., Tabagchali S. Transfer of macrolide-lincosamide-streptogramin B (MLS) resistance in Clostridium difficile is linked to a gene homologous with toxin A and is mediated by a conjugative transposon, Tn5398. J Antimicrob Chemother. 1995;35:305–315. doi: 10.1093/jac/35.2.305. [DOI] [PubMed] [Google Scholar]
- 24.Zaman S., Fitzpatrick M., Lindahl L., Zengel J. Novel mutations in ribosomal proteins L4 and L22 that confer erythromycin resistance in Escherichia coli. Mol Microbiol. 2007;66:1039–1050. doi: 10.1111/j.1365-2958.2007.05975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gregory S.T., Dahlberg A.E. Erythromycin resistance mutations in ribosomal proteins L22 and L4 perturb the higher order structure of 23S ribosomal RNA. J Mol Biol. 1999;289:827–834. doi: 10.1006/jmbi.1999.2839. [DOI] [PubMed] [Google Scholar]
- 26.Svenungsson B., Burman L.G., Jalakas-Pörnull K., Lagergren A., Struwe J., Åkerlund T. Epidemiology and molecular characterization of Clostridium difficile strains from patients with diarrhea: low disease incidence and evidence of limited cross-infection in a Swedish teaching hospital. J Clin Microbiol. 2003;41:4031–4037. doi: 10.1128/JCM.41.9.4031-4037.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eyre D.W., Cule M.L., Wilson D.J., Griffiths D., Vaughan A., O’Connor L. Diverse sources of C. difficile infection identified on whole-genome sequencing. N Engl J Med. 2013;26:1195–1205. doi: 10.1056/NEJMoa1216064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toepfer M., Magnusson C., Norén T., Hansen I., Iveroth P., Offenbartl K. Insidious and widespread outbreak of Clostridium difficile. Lakartidningen. 2014;111:24–27. [PubMed] [Google Scholar]
- 29.Coia J. What is the role of antimicrobial resistance in the new epidemic of Clostridium difficile? J Antimicrobial Agents. 2009;51:9–12. doi: 10.1016/S0924-8579(09)70009-3. [DOI] [PubMed] [Google Scholar]